Elevated glucose levels increase vascular calcification risk by disrupting extracellular pyrophosphate metabolism
- PMID: 39529124
- PMCID: PMC11555999
- DOI: 10.1186/s12933-024-02502-w
Elevated glucose levels increase vascular calcification risk by disrupting extracellular pyrophosphate metabolism
Abstract
Background: Vascular calcification is a major contributor to cardiovascular disease, especially diabetes, where it exacerbates morbidity and mortality. Although pyrophosphate is a recognized natural inhibitor of vascular calcification, there have been no prior studies examining its specific deficiency in diabetic conditions. This study is the first to analyze the direct link between elevated glucose levels and disruptions in extracellular pyrophosphate metabolism.
Methods: Rat aortic smooth muscle cells, streptozotocin (STZ)-induced diabetic rats, and diabetic human aortic smooth muscle cells were used to assess the effects of elevated glucose levels on pyrophosphate metabolism and vascular calcification. The techniques used include extracellular pyrophosphate metabolism assays, thin-layer chromatography, phosphate-induced calcification assays, BrdU incorporation for DNA synthesis, aortic smooth muscle cell viability and proliferation assays, and quantitative PCR for enzyme expression analysis. Additionally, extracellular pyrophosphate metabolism was examined through the use of radiolabeled isotopes to track ATP and pyrophosphate transformations.
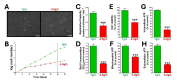
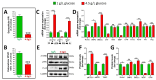
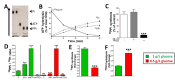
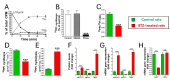
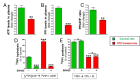
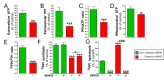
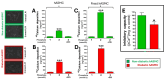
Results: Elevated glucose led to a significant reduction in extracellular pyrophosphate across all diabetic models. This metabolic disruption was marked by notable downregulation of both the expression and activity of ectonucleotide pyrophosphatase/phosphodiesterase 1, a key enzyme that converts ATP to pyrophosphate. We also observed an upregulation of ectonucleoside triphosphate diphosphohydrolase 1, which preferentially hydrolyzes ATP to inorganic phosphate rather than pyrophosphate. Moreover, tissue-nonspecific alkaline phosphatase activity was markedly elevated across all diabetic models. This shift in enzyme activity significantly reduced the pyrophosphate/phosphate ratio. In addition, we noted a marked downregulation of matrix Gla protein, another inhibitor of vascular calcification. The impaired pyrophosphate metabolism was further corroborated by calcification experiments across all three diabetic models, which demonstrated an increased propensity for vascular calcification.
Conclusions: This study demonstrated that diabetes-induced high glucose disrupts extracellular pyrophosphate metabolism, compromising its protective role against vascular calcification. These findings identify pyrophosphate deficiency as a potential mechanism in diabetic vascular calcification, highlighting a new therapeutic target. Strategies aimed at restoring or enhancing pyrophosphate levels may offer significant potential in mitigating cardiovascular complications in diabetic patients, meriting further investigation.
Keywords: ATP; Aging; Diabetes; Phosphate; Pyrophosphate; Vascular calcification.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Cloete L. Diabetes mellitus: an overview of the types, symptoms, complications and management. Nurs Stand R Coll Nurs G B 1987. 2022;37(1):61–6. - PubMed
-
- Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20(10):685–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

