Distinct neutrophil effector functions in response to different isolates of Leishmania aethiopica
- PMID: 39529155
- PMCID: PMC11555981
- DOI: 10.1186/s13071-024-06489-x
Distinct neutrophil effector functions in response to different isolates of Leishmania aethiopica
Abstract
Background: In Ethiopia, cutaneous leishmaniasis is mainly caused by Leishmania (L.) aethiopica parasites and presents in three main clinical forms. It is still not clear if the host immune response plays a role in the development of these different presentations. Since neutrophils are likely to be one of the first immune cells present at the site of the sand fly bite, we set up an in vitro model of infection of neutrophils with L. aethiopica and assessed some of the main neutrophil effector functions: association with and internalisation of parasites, apoptosis and ROS production. We used three freshly isolated clinical isolates and one isolate that has been kept in culture for decades.
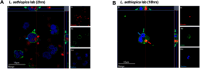
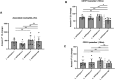
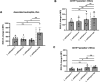
Results: Our results showed by flow cytometry that all four L. aethiopica isolates had the ability to associate with neutrophils. The three clinical isolates of L. aethiopica associated more efficiently with neutrophils than the long-term cultured L. aethiopica. At 18 h, two distinct populations of neutrophils were identified that associated with L. aethiopica, CD15high and CD15low neutrophils. Confocal microscopy demonstrated that all isolates can be internalised. Our results also showed that all parasites induced apoptosis in L. aethiopica-associated neutrophils. Moreover, our results showed that after 2 h, L. aethiopica-associated neutrophils upregulated their production of ROS, but to a greater extent with the long-term cultured L. aethiopica. After 18 h of incubation, CD15lowparasite+ showed an impaired ability to produce ROS compared to CD15highparasite+.
Conclusions: Using this in vitro model, our results show that different L. aethiopica parasite isolates, most notably long-term cultured parasites, had differential effects on neutrophil effector functions.
Keywords: Leishmania aethiopica; Apoptosis; Neutrophils; Phagocytosis; ROS.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Ruiz-Postigo JA, Jain S, Madjou S, Virrey Agua JF, Maia-Elkhoury AN, Valadas S, et al. Global leishmaniasis surveillance, 2022: assessing trends over the past 10 years. Wkly Epidemiol Rec. 2023;40:471–87.
-
- Yizengaw E, Takele Y, Franssen SU, Gashaw B, Yimer M, Abera A, et al. Parasite genetic variation and systemic immune responses are not associated with different clinical presentations of cutaneous leishmaniasis caused by Leishmania aethiopica. BioRxiv. 2024. 10.1101/2024.04.19.590259. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

