3D-environment and muscle contraction regulate the heterogeneity of myonuclei
- PMID: 39529179
- PMCID: PMC11552141
- DOI: 10.1186/s13395-024-00359-x
3D-environment and muscle contraction regulate the heterogeneity of myonuclei
Abstract
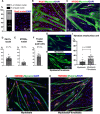
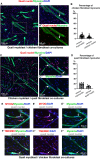
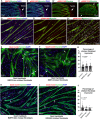
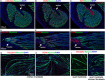
Skeletal muscle formation involves tight interactions between muscle cells and associated connective tissue fibroblasts. Every muscle displays the same type of organisation, they are innervated in the middle and attached at both extremities to tendons. Myonuclei are heterogeneous along myotubes and regionalised according to these middle and tip domains. During development, as soon as myotubes are formed, myonuclei at muscle tips facing developing tendons display their own molecular program. In addition to molecular heterogeneity, a subset of tip myonuclei has a fibroblastic origin different to the classical somitic origin, highlighting a cellular heterogeneity of myonuclei in foetal myotubes. To gain insights on the functional relevance of myonucleus heterogeneity during limb development, we used 2D culture and co-culture systems to dissociate autonomous processes (occurring in 2D-cultures) from 3D-environment of tissue development. We also assessed the role of muscle contraction in myonucleus heterogeneity in paralysed limb muscles. The regionalisation of cellular heterogeneity was not observed in 2D cell culture systems and paralyzed muscles. The molecular signature of MTJ myonuclei was lost in a dish and paralysed muscles indicating a requirement of 3D-enviroment and muscle contraction for MTJ formation. Tip genes that maintain a regionalized expression at myotube tips in cultures are linked to sarcomeres. The behaviour of regionalized markers in cultured myotubes and paralyzed muscles allows us to speculate whether the genes intervene in myogenesis, myotube attachment or MTJ formation.
Keywords: Cell cultures; Chicken; Embryos; Fibroblast; Heterogeneity; Immobilization; Limbs; Myoblast; Myonuclei; Myotendinous junction; Quail; Regionalisation.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Chevallier A, Kieny M, Mauger A. Limb-somite relationship: origin of the limb musculature. J Embryol Exp Morphol. 1977;41:245–58. - PubMed
-
- Christ B, Jacob HJ, Jacob M. Experimental analysis of the origin of the wing musculature in avian embryos. Anat Embryol (Berl). 1977;150:171–86. - PubMed
-
- Helmbacher F, Stricker S. Tissue cross talks governing limb muscle development and regeneration. Semin Cell Dev Biol. 2020;104:14–30. - PubMed
-
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–32. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

