Ultrasound-Defined Sarcopenia Independently Predicts Acute Decompensation in Advanced Chronic Liver Disease
- PMID: 39529225
- PMCID: PMC11634521
- DOI: 10.1002/jcsm.13630
Ultrasound-Defined Sarcopenia Independently Predicts Acute Decompensation in Advanced Chronic Liver Disease
Abstract
Background: It has been shown that in patients with liver cirrhosis, sarcopenia is a predictor of acute decompensation (AD), acute-on-chronic liver failure (ACLF) and death. However, computer tomography (CT), as a suggested standard method for diagnosing sarcopenia, is resource intensive and involves radiation exposure. Therefore, in this study, we evaluate the muscle thickness of quadriceps femoris measured by ultrasound (US) as a prognostic parameter for AD and all-cause mortality in chronic liver disease.
Methods: Sixty-three patients with chronic liver disease and signs of portal hypertension were analysed in this prospective monocentric study for the occurrence of acute decompensation such as hepatic encephalopathy, ascites, haemorrhage and liver-related death within 1 year. We assessed muscle thickness at three different heights in terms of suitability as a predictor.
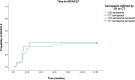
Results: Among all 63 patients, 15 patients experienced acute decompensation, and 9 patients died due to liver-related death. We found the upper third of the muscle, measured without applying pressure with the transducer, to be the most significant for predicting AD/ACLF [AUC 0.739 (confidence interval (CI) 0.604-0.874, p = 0.006]. A cut-off value of US-defined muscle thickness standardized per height for identifying sarcopenia was determined (1.83 cm/m). Patients with US-defined sarcopenia showed significantly higher rates of AD (38.9% vs. 3.7%, p = 0.001) and all-over 1-year mortality (27.8% vs. 3.7%, p = 0.013). The mean AD free survival time is 8.3 months (95% CI 6.6-9.9) for sarcopenic patients and 11.8 months (95% CI 11.0-12.6) for the non-sarcopenic cohorts. Corresponding CT analysis displayed similar results for AD free survival for both groups (40% AD rate in the sarcopenic group vs. 7% AD rate in the non-sarcopenic group, p = 0.001). The risk for AD was significantly higher in the sarcopenic cohort compared with those without sarcopenia in both US and CT (US: HR 16.6; p = 0.009; 95% CI 2.0-136.0; CT: HR 8.7; p = 0.017; 95% CI 1.5-51.0). CT and US displayed a moderate agreement (p = 0.006; κ = 0.379).
Conclusions: Sarcopenia classification based on US measurements is shown to be an independent predictor of AD occurrence within 1 year. This pilot study is the first to suggest that screening for sarcopenia by ultrasonography may be useful for risk assessment in patients with chronic liver disease and signs of portal hypertension.
Keywords: ACLF; acute decompensation; chronic liver disease; cirrhosis; malnutrition; portal hypertension; sarcopenia; skeletal muscle index; ultrasound.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Hanai T., Shiraki M., Nishimura K., et al., “Sarcopenia Impairs Prognosis of Patients With Liver Cirrhosis,” Nutrition 31, no. 1 (2015): 193–199. - PubMed
-
- Tantai X., Liu Y., Yeo Y. H., et al., “Effect of Sarcopenia on Survival in Patients With Cirrhosis: A Meta‐Analysis,” Journal of Hepatology 76, no. 3 (2022): 588–599. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

