Low Infection Rates With Long-Term Dupilumab Treatment in Patients Aged 6 Months to 5 Years: An Open-Label Extension Study
- PMID: 39529307
- PMCID: PMC11950805
- DOI: 10.1111/pde.15781
Low Infection Rates With Long-Term Dupilumab Treatment in Patients Aged 6 Months to 5 Years: An Open-Label Extension Study
Abstract
Objective: To evaluate long-term infection rates in children aged 6 months to 5 years with moderate-to-severe atopic dermatitis (AD) treated with dupilumab.
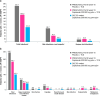
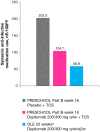
Methods: This was a post hoc analysis of an ongoing open-label extension (OLE) study of dupilumab. Pediatric patients aged 6 months to 5 years with moderate-to-severe AD who had previously taken part in the LIBERTY AD PRESCHOOL phase 2 and 3 clinical trials received weight-based subcutaneous dupilumab every 2 or 4 weeks. Exposure-adjusted infection rates after a median dupilumab exposure of 52 weeks are compared with data from the earlier randomized, placebo-controlled, 16-week LIBERTY AD PRESCHOOL phase 3 trial.
Results: Infection rates were overall lower in the OLE study compared with the dupilumab and placebo groups in the earlier 16-week trial, including total infections (101.0 patients/100 patient-years [PY]), nonherpetic skin infections (22.7 patients/100PY), herpetic infections (7.3 patients/100PY), and nonskin infections (92.9 patients/100PY). The frequency of severe and serious infections was low (3.1 patients/100PY), compared with 17.1 placebo-treated patients/100PY and 0 dupilumab-treated patients in the earlier 16-week trial, and no infections leading to treatment discontinuation were observed. Systemic anti-infective medication use (58.9 patients/100PY) was lower in the OLE study compared with both the dupilumab and placebo groups in the 16-week trial.
Conclusion: Overall, reduced infection rates are observed in infants and young children with moderate-to-severe AD treated with dupilumab long-term, supporting the known safety profile of dupilumab.
Keywords: atopic dermatitis; dupilumab; eczema; infections; pediatric dermatology.
© 2024 Regeneron Pharmaceuticals Inc. Sanofi and The Author(s). Pediatric Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Paller reported serving as an investigator, consultant, and/or data and safety monitoring board member for AbbVie, Abeona Therapeutics, Amryt Pharma, Azitra, BioCryst, BMS, Boehringer Ingelheim, Castle Creek Biosciences, Catawba Research, Dermavant, Eli Lilly, Galderma, Incyte, InMed Pharmaceuticals, Janssen, Krystal Biotech, LEO Pharma, Novartis, Regeneron Pharmaceuticals Inc., Sanofi, Seanergy, TWi Biotechnology, and UCB. Dr. Ramien reported serving as a consultant, speaker, and/or investigator for AbbVie, Eli Lilly, LEO Pharma, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi. Dr. Cork reported serving as an investigator and/or consultant for AbbVie, Astellas Pharma, Boots, Dermavant, Galapagos, Galderma, Hyphens Pharma, Johnson & Johnson, LEO Pharma, L'Oréal, Menlo Therapeutics, Novartis, Oxagen, Pfizer, Procter & Gamble, Reckitt Benckiser, Regeneron Pharmaceuticals Inc., and Sanofi. Dr. Simpson has received personal fees from AbbVie, Advances in Cosmetic and Medical Dermatology Hawaii, Amgen, AOBiome, Arcutis Biotherapeutics, Arena Pharmaceuticals, Aslan Pharmaceuticals, BMS, Boehringer Ingelheim, Boston Consulting Group, Collective Acumen, CorEvitas, Dermira, Eli Lilly, Evelo Biosciences, Evidera, Excerpta Medica, Forté Biosciences, Fraunhofer, Galderma, GSK, Incyte, Janssen, Johnson & Johnson, Kyowa Kirin, LEO Pharma, Maui Derm, Medscape, Merck, MJH Life Sciences, MLG Operating, Pfizer, Physicians World, PRImE, Regeneron Pharmaceuticals Inc., Revolutionizing Atopic Dermatitis, Roivant Sciences, Sanofi, Trevi Therapeutics, Valeant, Vindico Medical Education, and WebMD; and received grants from (or undertook a principal investigator role with) AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Creek Biosciences, CorEvitas, Dermavant, Dermira, Eli Lilly, Incyte, Kymab, Kyowa Kirin, National Jewish Health, LEO Pharma, Pfizer, Regeneron Pharmaceuticals Inc., Sanofi, and Target RWE. Dr. Wine Lee has acted as an advisory board member consultant, investigator, data and safety monitoring board member, and/or speaker for AbbVie, Amgen, Amryt Pharma, Arcutis Biotherapeutics, BMS, Castle Creek Biosciences, Celgene, Eli Lilly, Galderma, Incyte, Kimberly‐Clark, Krystal Biotech, Mayne Pharma, Novartis, Pfizer, Pyramid Biosciences, Regeneron Pharmaceuticals Inc., Sanofi, Target Pharma, Trevi Therapeutics, and UCB. Dr. Eichenfield reported receiving honoraria for consulting services and/or research support from AbbVie, Amgen, Arcutis, Aslan Pharmaceuticals, Bausch, BMS, Castle Biosciences, Dermavant, Eli Lilly, Forté Pharma, Galderma, Incyte, Novartis, Otsuka, Pfizer, Regeneron Pharmaceuticals Inc., Sanofi, and UCB. Drs Khokhar, Chen, and Cyr are employees and shareholders of Regeneron Pharmaceuticals Inc. Drs Coleman, Gherardi, and Zhang are employees of and may hold stock and/or stock options in Sanofi.
Figures
Similar articles
-
Dupilumab Provides Acceptable Safety and Sustained Efficacy for up to 4 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis.Am J Clin Dermatol. 2022 May;23(3):393-408. doi: 10.1007/s40257-022-00685-0. Epub 2022 May 3. Am J Clin Dermatol. 2022. PMID: 35503163 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Upadacitinib versus Dupilumab Treatment for Moderate-to-Severe Atopic Dermatitis in Four Body Regions: Analysis from the Heads Up Study.Dermatology. 2025;241(1):10-18. doi: 10.1159/000542275. Epub 2024 Oct 30. Dermatology. 2025. PMID: 39476813 Free PMC article. Clinical Trial.
-
Systemic treatments for eczema: a network meta-analysis.Cochrane Database Syst Rev. 2020 Sep 14;9(9):CD013206. doi: 10.1002/14651858.CD013206.pub2. Cochrane Database Syst Rev. 2020. PMID: 32927498 Free PMC article.
-
Risk of infection in patients with atopic dermatitis treated with dupilumab: A meta-analysis of randomized controlled trials.J Am Acad Dermatol. 2018 Jan;78(1):62-69.e1. doi: 10.1016/j.jaad.2017.09.052. Epub 2017 Oct 4. J Am Acad Dermatol. 2018. PMID: 28987493
-
Dupilumab Efficacy in Children with Atopic Dermatitis with Different Phenotypes and Endotypes: A Case Series.Adv Ther. 2025 Jul;42(7):3186-3206. doi: 10.1007/s12325-025-03150-6. Epub 2025 May 8. Adv Ther. 2025. PMID: 40338484 Free PMC article. Clinical Trial.

