Blood flow-induced angiocrine signals promote organ growth and regeneration
- PMID: 39529434
- PMCID: PMC11755702
- DOI: 10.1002/bies.202400207
Blood flow-induced angiocrine signals promote organ growth and regeneration
Erratum in
-
Correction.Bioessays. 2025 Apr;47(4):e202500017. doi: 10.1002/bies.202500017. Epub 2025 Feb 24. Bioessays. 2025. PMID: 39989050 Free PMC article. No abstract available.
Abstract
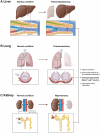
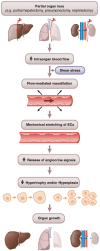
Recently, we identified myeloid-derived growth factor (MYDGF) as a blood flow-induced angiocrine signal that promotes human and mouse hepatocyte proliferation and survival. Here, we review literature reporting changes in blood flow after partial organ resection in the liver, lung, and kidney, and we describe the angiocrine signals released by endothelial cells (ECs) upon blood flow alterations in these organs. While hepatocyte growth factor (HGF) and MYDGF are important angiocrine signals for liver regeneration, by now, angiocrine signals have also been reported to stimulate hyperplasia and/or hypertrophy during the regeneration of lungs and kidneys. In addition, angiocrine signals play a critical role in tumor growth. Understanding the mechano-elastic properties and flow-mediated alterations in the organ-specific microvasculature is crucial for therapeutic approaches to maintain organ health and initiate organ renewal.
Keywords: Myeloid‐derived growth factor (MYDGF); angiocrine signals; blood flow; liver; organ growth; regeneration.
© 2024 The Author(s). BioEssays published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration.Nature. 2010 Nov 11;468(7321):310-5. doi: 10.1038/nature09493. Nature. 2010. PMID: 21068842 Free PMC article.
-
Identification of myeloid-derived growth factor as a mechanically-induced, growth-promoting angiocrine signal for human hepatocytes.Nat Commun. 2024 Feb 5;15(1):1076. doi: 10.1038/s41467-024-44760-y. Nat Commun. 2024. PMID: 38316785 Free PMC article.
-
Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival.Nature. 2018 Oct;562(7725):128-132. doi: 10.1038/s41586-018-0522-3. Epub 2018 Sep 26. Nature. 2018. PMID: 30258227
-
Angiocrine signaling in the hepatic sinusoids in health and disease.Am J Physiol Gastrointest Liver Physiol. 2016 Aug 1;311(2):G246-51. doi: 10.1152/ajpgi.00118.2016. Epub 2016 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27288423 Free PMC article. Review.
-
Developmental angiocrine diversification of endothelial cells for organotypic regeneration.Dev Cell. 2021 Nov 22;56(22):3042-3051. doi: 10.1016/j.devcel.2021.10.020. Dev Cell. 2021. PMID: 34813766 Review.
Cited by
-
Single cell transcriptomics of human kidney organoid endothelium reveals vessel growth processes and arterial maturation upon transplantation.NPJ Regen Med. 2025 Jul 1;10(1):32. doi: 10.1038/s41536-025-00418-x. NPJ Regen Med. 2025. PMID: 40595660 Free PMC article.
References
-
- Michalopoulos, G. K. , & Bhushan, B. (2021). Liver regeneration: Biological and pathological mechanisms and implications. Nature Reviews Gastroenterology & Hepatology, 18, 40–55. - PubMed
-
- Fernández, L. G. , Isbell, J. M. , Jones, D. R. , & Laubach, V. E. (2012). Compensatory lung growth after pneumonectomy. In Guerreiro Cardoso P. F. (Ed), Topics in thoracic surgery (pp. 409–426). IntechOpen.
-
- Rojas‐Canales, D. M. , Li, J. Y. , Makuei, L. , & Gleadle, J. M. (2019). Compensatory renal hypertrophy following nephrectomy: When and how? Nephrology, 24, 1225–1232. - PubMed
-
- Große‐Segerath, L. , & Lammert, E. (2021). Role of vasodilation in liver regeneration and health. Biological Chemistry, 402, 1009–1019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

