HPV16/18 antibodies 16-years after single dose of bivalent HPV vaccination: Costa Rica HPV vaccine trial
- PMID: 39529529
- PMCID: PMC11555268
- DOI: 10.1093/jncimonographs/lgae032
HPV16/18 antibodies 16-years after single dose of bivalent HPV vaccination: Costa Rica HPV vaccine trial
Abstract
Background: The Costa Rica HPV Vaccine Trial provided initial evidence that 1 dose of the bivalent human papillomavirus (HPV) vaccine induces stabilizing antibody levels that may provide extended protection against HPV-16/18 infections. We report antibody seropositivity and stability 11 to 16 years after vaccination.
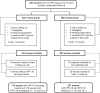
Methods: We invited a random subset of Costa Rica HPV Vaccine Trial participants (n = 398) who had received 3 doses and all women (n = 203) who had received 1 dose at 18 to 25 years of age to follow-up visits 11, 14, and 16 years after vaccination. We calculated HPV-16 and HPV-18 seropositivity and assessed change in enzyme-linked immunosorbent assay antibody levels 11 to 16 years after vaccination among 500 participants.
Results: By year 16, 99.4% (95% confidence interval [CI] = 96.8% to 100.0%) and 100.0% (95% CI = 98.9% to 100.0%) of 1-dose and 3-dose recipients, respectively, were HPV-16 seropositive and 98.8% (95% CI = 95.9% to 99.9%) and 100% (95% CI = 98.9% to 100.0%) of 1-dose and 3-dose recipients, respectively, were HPV-18 seropositive. Between years 11 and 16, women who had received 3 doses had a small but statistically significant decrease in the geometric mean concentration for HPV-16 of ‒12.4% (95% CI = ‒16.3% to ‒8.4%) and HPV-18 of ‒13.4% (95% CI = ‒17.2% to ‒9.4%). Among women who had received 1 dose, the decrease was statistically significant for HPV-16 at ‒8.9 (95% CI = ‒14.2% to ‒3.1%) but nonsignificant for HPV-18. Geometric mean concentration ratios of 3:1 dose (year 16) were 3.0 and 2.2 for HPV-16 and HPV-18, respectively.
Conclusions: HPV-16/18 seropositivity remained exceedingly high 16 years after vaccination. Over 5 years, small declines in antibodies were observed. Women should have protection for at least 20 years and likely much longer at the observed rate of decline.
Published by Oxford University Press 2024.
Conflict of interest statement
John T. Schiller and Douglas R. Lowy report that they are named inventors on US government–owned HPV vaccine patents, with expired licenses to GlaxoSmithKline and Merck. The other authors declare that they have no conflicts of interest.
Investigators in the CVT Group: Bernal Cortés, Paula González (deceased), Rolando Herrero, Silvia E. Jiménez, Carolina Porras, Ana Cecilia Rodríguez (Agencia Costarricense de Investigaciones Biomédicas, formerly Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica); Allan Hildesheim, Aimée R. Kreimer, Douglas R. Lowy, Mark Schiffman, John T. Schiller, Mark Sherman, Sholom Wacholder (deceased) (US National Cancer Institute, Bethesda, MD); Ligia A. Pinto, Troy J. Kemp (Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research, Frederick, MD); Mary K. Sidawy, (Georgetown University, Washington, DC); Wim Quint (deceased), Leen-Jan van Doorn, Linda Struijk (DDL Diagnostic Laboratory, the Netherlands); Joel M. Palefsky, Teresa M. Darragh (University of California, San Francisco, San Francisco, CA); and Mark H. Stoler (University of Virginia, Charlottesville, VA).
We dedicate this work to the memory of our beloved colleague and friend Paula Gonzalez, the principal investigator of the CVT long-term follow-up study. We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions of Carlos Avila, Loretto Carvajal, Rebeca Ocampo, Cristian Montero, and Diego Guillen. In the United States, we extend our appreciation to the team from Information Management Services responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr Diane Solomon (CVT: medical monitor and quality control pathologist) for her invaluable contributions to the design and conduct of the trial and Nora Macklin and Kate Torres for their expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomized, blinded phase of our study (Chair Steve Self , Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group, who have contributed to the success of our efforts over the years (Henriette Raventós, Chair, Diane Davey, Gypsyamber D’Souza, Anne Gershon, Silvia Lara, Wasima Rida, Richard Roden, Maria del Rocío Sáenz Madrigal, and Margaret Stanley). We thank the Division of Cancer Epidemiology and Genetics Cancer Genomics Research Laboratory and acknowledge the support from Belynda Hicks, Casey Dagnall, and Amy Hutchinson.
Preliminary findings for this analysis were presented at the International Papillomavirus Conference in Washington DC, in April 2023.
Figures


References
-
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva: World Health Organization; 2020.
-
- Bruni L, Saura-Lazaro A, Montoliu A, et al.HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. 2021;155:106925. - PubMed
-
- World Health Organization = Organisation mondiale de la Santé. Weekly Epidemiological Record, 2022, vol. 97, 50 [full issue]. Weekly Epidemiol Rec = Relevé Épidémiologique Hebdomadaire. 2022;97(50):645-672.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

