A High-Throughput Microphysiological Liver Chip System to Model Drug-Induced Liver Injury Using Human Liver Organoids
- PMID: 39529647
- PMCID: PMC11550169
- DOI: 10.1016/j.gastha.2024.08.004
A High-Throughput Microphysiological Liver Chip System to Model Drug-Induced Liver Injury Using Human Liver Organoids
Abstract
Background and aims: Drug-induced liver injury (DILI) is a major failure mode in pharmaceutical development. This study aims to address the limitations of existing preclinical models by assessing a high-throughput, microfluidic liver-on-a-chip system, termed "Curio Barrier Liver Chips," and its capacity to recapitulate the effects of chronic hepatotoxic drug treatment through metabolic and phenotypic characterization.
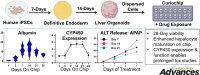
Methods: Curio Barrier liver chips (Curiochips), fabricated in an 8 × 2 well configuration, were utilized to establish three dimensional liver organoid cultures. Human-induced pluripotent stem cells were differentiated into human liver organoids, and their viability, liver-specific functions, and pharmacological responses were assessed over 28 days.
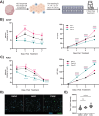
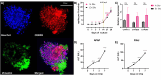
Results: The Curiochips successfully maintained liver physiology and function, showing strong albumin secretion and cytochrome (CYP) P450 activities for 28 days. Unlike traditional models requiring millimolar drug concentrations to detect hepatotoxicity, this platform showed increased sensitivity for acetaminophen and fialuridine at micromolar concentrations. In situ differentiation of foregut spheroids to liver organoids was also achieved, further simplifying the establishment of liver chips. Furthermore, the chips demonstrated viability, function, and DILI responsiveness for 28 days, making this an improved model for studying idiosyncratic DILI with prolonged drug exposure and high-throughput capabilities compared to other available systems or primary human hepatocytes.
Conclusion: The Curiochips offer an advanced, miniaturized in vitro model for early-stage drug development and a sensitive, responsive, and cost-effective means to detect direct hepatotoxicity. Induced pluripotent stem cell liver organoids, in conjunction with the Curiochip, deliver a high-throughput platform with robust functionality and pharmacological responsiveness that make it a promising tool for improving the prediction and understanding of DILI risk prediction, especially with prolonged drug exposure. The model also opens new avenues for research in other chronic liver diseases.
Keywords: Curiochips; DILI Model; Drug-Induced Liver Injury; Hepatotoxicity; High-Throughput Screening; Microfluidic Liver Chips; Microphysiologic Liver System; iPSC-Derived Human Liver organoids.
© 2024 The Authors.
Figures


References
-
- Stevens J.L., Baker T.K. The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov Today. 2009;14(3–4):162–167. - PubMed
-
- Wysowski D.K., Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969-2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165(12):1363–1369. - PubMed
-
- Bakke O.M., Manocchia M., de Abajo F., et al. Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: a regulatory perspective. Clin Pharmacol Ther. 1995;58(1):108–117. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

