Durability and Efficacy of Faricimab in Treatment-Resistant Retinal Edema Utilizing "Real-World" Dosing Regimens
- PMID: 39529696
- PMCID: PMC11554410
- DOI: 10.1155/2024/8583348
Durability and Efficacy of Faricimab in Treatment-Resistant Retinal Edema Utilizing "Real-World" Dosing Regimens
Abstract
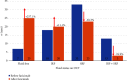
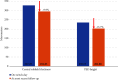
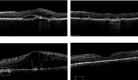
Purpose: To retrospectively analyze clinical outcomes of patients with "treatment-resistant" neovascular age-related macular degeneration or diabetic macular edema who were switched to intravitreal faricimab injections (IFIs) using a "real-world" treat-and-extend (TAE) protocol. Methods: Seventy-one eyes from 62 patients receiving antivascular endothelial growth factor injections were evaluated before and after switching to IFI. Demographic and clinical data were collected. Primary endpoints were treatment interval extension and presence of intraretinal or subretinal fluid on spectral-domain optical coherence tomography (OCT) after switching to IFI. Secondary endpoints included best-corrected visual acuity, average OCT central subfield thickness, and presence of a pigment epithelium detachment and pigment epithelium detachment height. Results: The average treatment interval after switching to IFI significantly increased from 37.6 ± 10.8 days to 45.2 ± 16.6 days (p = 0.0016). At the last follow-up, 35% of eyes were able to achieve a fluid-free status post-IFI. A chi-square test of independence validated this finding by showing a significant difference in the OCT findings trending towards less or no fluid on follow-up (X 2 [3, N = 71] = 13.0705; p = 0.0003). The average central subfield thickness decreased from 327.2 ± 89.1 μm to 294.8 ± 86.5 μm (p = 0.0294). Best-corrected visual acuity, intraocular pressure, pigment epithelium detachment presence, and height had no significant difference after switching to IFI. Conclusions: In "treatment-resistant" patients receiving anti-VEGF therapy for neovascular age-related macular degeneration or diabetic macular edema, switching to IFI in a "real-world" TAE protocol led to statistically significant improvements in treatment interval and retinal fluid on spectral domain OCT.
Keywords: faricimab; intravitreal injection; treat and extend; treatment-resistant edema.
Copyright © 2024 Shravan V. Savant et al.
Conflict of interest statement
S.V.S., J.T.K., F.B., J.C., J.M., G.B., and K.K.M. declare no conflicts of interest. D.J.R.: consultant for Beaver-Visitec International, Inc.; consultant for Regeneron Pharmaceuticals Inc.
Figures
References
LinkOut - more resources
Full Text Sources

