High-dose Probiotic Mix of Lactobacillus spp., Bifidobacterium spp., Bacillus coagulans, and Saccharomyces boulardii to Prevent Antibiotic-associated Diarrhea in Adults: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial (SPAADA)
- PMID: 39529939
- PMCID: PMC11551610
- DOI: 10.1093/ofid/ofae615
High-dose Probiotic Mix of Lactobacillus spp., Bifidobacterium spp., Bacillus coagulans, and Saccharomyces boulardii to Prevent Antibiotic-associated Diarrhea in Adults: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial (SPAADA)
Abstract
Background: Probiotics have been used to prevent antibiotic-associated diarrhea (AAD), but practical guidelines are sparse. This trial evaluated the efficacy and safety of a high-dose, multistrain probiotic mix (Sinquanon), specially designed for prevention of AAD in adults.
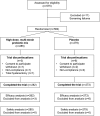
Methods: A phase IV, multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial was conducted over 5 months. Participants receiving broad-spectrum antibiotics were administered the specialized probiotic mix or placebo from the first dose of antibiotics until 14 days after the last antibiotic dose. The primary outcome measure was the incidence of AAD.
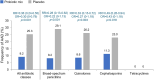
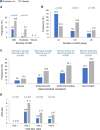
Results: In total, 564 participants were randomized (probiotic mix: 285; placebo: 279), of which 9 participants discontinued the trial early (probiotic mix: 3; placebo: 6), had no efficacy data, and were excluded from the efficacy analysis. The 555 remaining participants completed the trial and were included in the efficacy analysis (probiotic mix: 282; placebo: 273). AAD occurred less frequently in the studied probiotic mix versus placebo group (9.2% vs 25.3%, P < .001), resulting in an absolute risk reduction of 16% and a number needed to treat of 6 (95% confidence interval, 4.55-10.49). A significant improvement in the average gastrointestinal quality of life in the studied probiotic mix versus placebo group was also observed. There were no clinically relevant differences in the incidence of adverse events between the studied probiotic mix and the placebo group.
Conclusions: The specially designed high-dose, multistrain probiotic mix (Sinquanon) demonstrated to be beneficial compared with placebo in the prevention of AAD in adults who received broad-spectrum antibiotics.
Clinicaltrialsgov identifier and url: NCT05607056; https://classic.clinicaltrials.gov/ct2/show/NCT05607056.
Keywords: antibiotic-associated diarrhea; high-dose; multi-strain; probiotic; randomized controlled trial.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors have delivered scientific lectures on a given problem for various pharmaceutical companies, including Neopharm Bulgaria.
Figures
Comment in
-
High-Dose Probiotic Mix of Lactobacillus Spp, Bifidobacterium Spp, Bacillus coagulans, and Saccharomyces boulardii to Prevent Antibiotic-Associated Diarrhea in Adults: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial (SPAADA).Open Forum Infect Dis. 2025 May 27;12(6):ofaf316. doi: 10.1093/ofid/ofaf316. eCollection 2025 Jun. Open Forum Infect Dis. 2025. PMID: 40491930 Free PMC article. No abstract available.
References
-
- McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol 2008; 3:563–78. - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

