Brainstem opioid peptidergic neurons regulate cough reflexes in mice
- PMID: 39529953
- PMCID: PMC11551472
- DOI: 10.1016/j.xinn.2024.100721
Brainstem opioid peptidergic neurons regulate cough reflexes in mice
Abstract
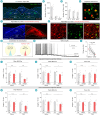
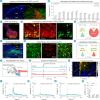
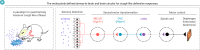
Cough is a vital defensive reflex for expelling harmful substances from the airway. The sensory afferents for the cough reflex have been intensively studied. However, the brain mechanisms underlying the cough reflex remain poorly understood. Here, we developed a paradigm to quantitatively measure cough-like reflexes in mice. Using this paradigm, we found that prodynorphin-expressing (Pdyn+) neurons in the nucleus of the solitary tract (NTS) are critical for capsaicin-induced cough-like reflexes. These neurons receive cough-related neural signals from Trpv1+ vagal sensory neurons. The activation of Pdyn+ NTS neurons triggered respiratory responses resembling cough-like reflexes. Among the divergent projections of Pdyn+ NTS neurons, a glutamatergic pathway projecting to the caudal ventral respiratory group (cVRG), the canonical cough center, was necessary and sufficient for capsaicin-induced cough-like reflexes. These results reveal that Pdyn+ NTS neurons, as a key neuronal population at the entry point of the vagus nerve to the brainstem, initiate cough-like reflexes in mice.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources

