Accelerated innervation of biofabricated skeletal muscle implants containing a neurotrophic factor delivery system
- PMID: 39530055
- PMCID: PMC11550949
- DOI: 10.3389/fbioe.2024.1476370
Accelerated innervation of biofabricated skeletal muscle implants containing a neurotrophic factor delivery system
Abstract
Introduction: Volumetric muscle loss (VML) is one of the most severe and debilitating conditions in orthopedic and regenerative medicine. Current treatment modalities often fail to restore the normal structure and function of the damaged skeletal muscle. Bioengineered tissue constructs using the patient's own cells have emerged as a promising alternative treatment option, showing positive outcomes in fostering new muscle tissue formation. However, achieving timely and proper innervation of the implanted muscle constructs remains a significant challenge. In this study, we present a clinically relevant strategy aimed at enhancing and sustaining the natural regenerative response of peripheral nerves to accelerate the innervation of biofabricated skeletal muscle implants.
Methods: We previously developed a controlled-release neurotrophic factor delivery system using poly (lactic-co-glycolic acid) (PLGA) microspheres encapsulating ciliary neurotrophic factor (CNTF) and glial cell line-derived neurotrophic factor (GDNF). Here, we incorporate this neurotrophic factor delivery system into bioprinted muscle constructs to facilitate innervation in vivo.
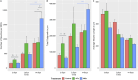
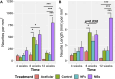
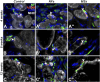
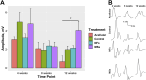
Results: Our results demonstrate that the neurotrophic factors released from the microspheres provide a chemical cue, significantly enhancing the neurite sprouting and functional innervation of the muscle cells in the biofabricated muscle construct within 12 weeks post-implantation.
Discussion: Our approach provides a clinically applicable treatment option for VML through accelerated innervation of biomanufactured muscle implants and subsequent improvements in functionality.
Keywords: CNTF; GDNF; bioengineered skeletal muscle; controlled-release delivery system; innervation; neurotrophic factors.
Copyright © 2024 Mashanov, Billman, Poerio, Kaufmann, Lai, Vaughan, Kim, Ju, Atala, Yoo and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

