A minority of proliferating human CD4+ T cells in antigen-driven proliferation assays are antigen specific
- PMID: 39530093
- PMCID: PMC11550966
- DOI: 10.3389/fimmu.2024.1491616
A minority of proliferating human CD4+ T cells in antigen-driven proliferation assays are antigen specific
Abstract
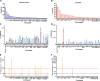
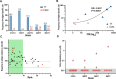
Antigen-driven T-cell proliferation is often measured using fluorescent dye dilution assays, such as the CFSE-based proliferation assay. Dye dilution assays have been powerful tools to detect human CD4+ T-cell responses, particularly against autoantigens. However, it is not known how many cells within the proliferating population are specific for the stimulating antigen. Here we determined the frequency of CD4+ T cells specific for the stimulating antigen within the antigen-responsive population of CFSE-based proliferation assays. We compared CD4+ T-cell responses to a type 1 diabetes autoantigen (proinsulin C-peptide) and to a vaccine antigen (tetanus toxoid). The TCRs expressed by antigen-responsive CD4+ T cells were sequenced, and their antigen specificity was tested functionally by expressing them in a reporter T-cell line. Responses to C-peptide were weak, but detectable, in PBMC from individuals with T1D, whereas responses to tetanus toxoid were much stronger. The frequency of antigen-specific CD4+ T cells correlated with the strength of the response to antigen in the proliferation assay. However, antigen-specific CD4+ T cells were rare among antigen-responsive CD4+ T cells. For C-peptide, an average frequency of 7.5% (1%-11%, n = 4) of antigen-responsive CD4+ T cells were confirmed to be antigen specific. In the tetanus-toxoid-stimulated cultures, on average, 45% (16%-78%, n = 5) of the antigen-responsive CD4+ T cells were tetanus toxoid specific. These data show that antigen-specific CD4+ T cells are a minority of the cells that proliferate in response to antigen and have important implications for in vitro CD4+ T-cell proliferation assays.
Keywords: C-peptide; CD4+ T cell; CFSE assay; antigen specific T cell; autoimmunity; clonal expansion; proliferation; tetanus toxoid.
Copyright © 2024 Bhattacharjee, Pakusch, Lacorcia, Chiu, Liu, Tresoldi, Foster, King, Cameron and Mannering.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor MN declared a past co-authorship with the author SM.
Figures


References
-
- Mannering SI, McKenzie JL, Fearnley DB, Hart DN. HLA-DR1-restricted bcr-abl (b3a2)-specific CD4+ T lymphocytes respond to dendritic cells pulsed with b3a2 peptide and antigen-presenting cells exposed to b3a2 containing cell lysates. Blood. (1997) 90:290–7. doi: 10.1182/blood.V90.1.290.290_290_297 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

