Immunomodulatory effects of cysteamine and its potential use as a host-directed therapy for tuberculosis
- PMID: 39530101
- PMCID: PMC11550979
- DOI: 10.3389/fimmu.2024.1411827
Immunomodulatory effects of cysteamine and its potential use as a host-directed therapy for tuberculosis
Abstract
Objective: Cysteamine, a drug approved to treat cystinosis, has been proposed as a host-directed therapy for M. tuberculosis (Mtb) and SARS-CoV-2. The impact of cysteamine on the immune responses has not been fully investigated. We aimed to in vitro evaluate the immunomodulatory effects of cysteamine on peripheral blood mononuclear cells (PBMCs) using the purified protein derivative (PPD) as a recall antigen, and an unspecific stimulus as staphylococcal enterotoxin B (SEB).
Methods: PBMCs isolated from subjects with tuberculosis infection (TBI), those with tuberculosis disease (TB), and healthy controls (HC) were in vitro stimulated with PPD or SEB and treated or not with cysteamine at different concentrations (50 µM-400 µM) for 6 hours (h) and 24 h. We evaluated the T helper1 (Th1) and T cytotoxic1 (Tc1) cell cytokine production by flow cytometry and immune-enzymatic assays. In HC, we also evaluated apoptosis and/or necrosis by flow cytometry.
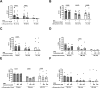
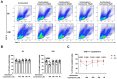
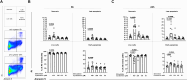
Results: We observed an immunomodulatory effect of cysteamine at 400 µM in PBMCs from TB and TBI subjects. It significantly reduced PPD-specific Th1 responses at 24 h and at 6 h (p=0.0004 and p=0.0009, respectively), and a similar non-significant trend was observed with cysteamine at 200 µM (p=0.06 at 24 h and p=0.14 at 6 h). Moreover, cysteamine at both 400 µM (p<0.0001 and p=0.0187 at 24 h, respectively, and p<0.0001 at 6 h for both) and 200 µM (p=0.0119 and p=0.0028 at 24 h and p=0.0028 and p=0.0003 at 6 h, respectively) significantly reduced SEB-induced Th1 and Tc1 responses. Furthermore, we found that cysteamine induced morphological lymphocyte changes and significantly reduced the lymphocyte percentage in a dose- and time-dependent manner. Cysteamine at 400 µM induced 8% late apoptosis and 1.6% necrosis (p<0.05) at 24 h. In contrast, despite significant differences from untreated conditions (p<0.05), cysteamine at 400 µM for 6 h induced approximately 1% late apoptosis and 0.1% necrosis in the cells.
Conclusions: High doses of cysteamine in vitro reduce the percentages of PPD- and SEB-induced Th1 and Tc1 cells and induce late apoptosis and necrosis. Differently, cysteamine at lower doses retains the immunomodulatory effect without affecting cell viability. These findings suggest cysteamine as a potential adjunct to antimicrobial regimens as in the TB or COVID-19 field, for its ability to reduce the inflammatory status.
Keywords: Ag-specific response; PPD-specific response; apoptosis; cysteamine; host-directed therapy; inflammation; necrosis; tuberculosis.
Copyright © 2024 Najafi-Fard, Farroni, Petrone, Altera, Salmi, Vanini, Cuzzi, Alonzi, Nicastri, Gualano, Palmieri, Piacentini and Goletti.
Conflict of interest statement
EN is member of the advisory board by Gilead, Lilly and Roche and received fees for educational training by Gilead, Lilly and Roche. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships related to this study that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- WHO . Global tuberculosis report 2023 (2023). Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa... (Accessed March 22, 2024).
-
- Goletti D, Aiello A, Tientcheu LD, Muefong C, Hu TH, Niewold P, et al. . Host–pathogen interactions in the context of tuberculosis infection and disease. European Respiratory Society (2023), 34–50. doi: 10.1183/2312508X.10024022 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

