Aquaporin-4 activation facilitates glymphatic system function and hematoma clearance post-intracerebral hemorrhage
- PMID: 39530196
- PMCID: PMC11662979
- DOI: 10.1002/glia.24639
Aquaporin-4 activation facilitates glymphatic system function and hematoma clearance post-intracerebral hemorrhage
Abstract
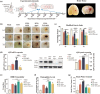
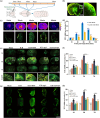
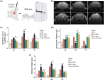
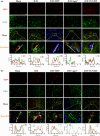
Efficient clearance of hematomas is crucial for improving clinical outcomes in patients with intracerebral hemorrhage (ICH). The glymphatic system, facilitated by aquaporin-4 (AQP4), plays a crucial role in cerebrospinal fluid (CSF) entry and metabolic waste clearance. This study examined the role of the glymphatic system in ICH pathology, with a focus on AQP4. Collagenase-induced ICH models were established, with AQP4 expression regulated through mifepristone as an agonist, TGN-020 as an inhibitor, and Aqp4 gene knockout. Fluorescence tracing and multimodal magnetic resonance imaging (MRI) were employed to observe glymphatic system functionality, hematoma, and edema volumes. Neurological deficit scoring was performed using the modified Garcia Scale. AQP4 expression was quantified using RT-qPCR and Western blotting, and cellular localization was explored using immunofluorescence. The brain tissue sections were examined for neuronal morphology, degenerative changes, and iron deposition. Three days post-ICH, the AQP4 agonist group showed increased AQP4 protein expression and perivascular polarization, decreased hemoglobin levels, and reduced iron deposition. Conversely, the inhibition group exhibited contrasting trends. AQP4 activation improved glymphatic system function, leading to a wider distribution, improved neurological function, and reduced hematoma. Pharmacological inhibition and genetic knockout of AQP4 have opposing effects. The glymphatic system, facilitated by AQP4, plays a crucial role in hematoma clearance following cerebral hemorrhage. Upregulation of AQP4 improves glymphatic system function, facilitates hematoma clearance, and promotes brain tissue recovery.
Keywords: AQP4; glymphatic system; hematoma clearance; intracerebral hemorrhage.
© 2024 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Figures

References
-
- Andres, K. H. , von Düring, M. , Muszynski, K. , & Schmidt, R. F. (1987). Nerve fibres and their terminals of the dura mater encephali of the rat. Anatomy and embryology, 175, 289–301. - PubMed
-
- Carol Kilkenny , Browne, W. J. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother, 1, 94–99. https://pubmed.ncbi.nlm.nih.gov/21350617/ - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

