Prioritization of Eleven-Nineteen-Leukemia Inhibitors as Orally Available Drug Candidates for Acute Myeloid Leukemia
- PMID: 39530508
- PMCID: PMC11613437
- DOI: 10.1021/acs.jmedchem.4c01337
Prioritization of Eleven-Nineteen-Leukemia Inhibitors as Orally Available Drug Candidates for Acute Myeloid Leukemia
Abstract
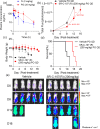
Acute myeloid leukemia (AML) is the second most prevalent and fatal form of leukemia. The growth of AML cells harboring oncogenic MLL rearrangements relies on the YEATS domain-containing protein ENL. Many small molecule inhibitors targeting ENL have been developed. To prioritize these inhibitors for in vivo studies, a NanoBRET system was introduced to evaluate their cellular permeability and potency. This screening identified inhibitor 13 as a promising candidate. This inhibitor has remarkable metabolic stability and potent antiproliferative effects on MLL-fusion leukemia cell lines. In AML-xenografted mice, inhibitor 13 significantly improved survival. Subsequent optimization efforts led to the development of SR-C-107 (R), which exhibited strong activity against AML both at the cellular level (CC50 (MOLM-13): 1.25 ± 0.18 μM; CC50 (MV4-11): 0.81 ± 0.15 μM) and in vivo. These findings establish SR-C-107 (R) as a compelling candidate for AML treatment and lay the groundwork for the development of next-generation AML inhibitors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Döhner H.; Estey E.; Grimwade D.; Amadori S.; Appelbaum F. R.; Büchner T.; Dombret H.; Ebert B. L.; Fenaux P.; Larson R. A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. 10.1182/blood-2016-08-733196. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

