Phase 2 Trial of Enfortumab Vedotin in Patients With Previously Treated Locally Advanced or Metastatic Urothelial Carcinoma in China
- PMID: 39530574
- PMCID: PMC11555717
- DOI: 10.1002/cam4.70368
Phase 2 Trial of Enfortumab Vedotin in Patients With Previously Treated Locally Advanced or Metastatic Urothelial Carcinoma in China
Abstract
Background: Enfortumab vedotin, a fully human monoclonal antibody-drug conjugate (ADC) directed to Nectin-4, prolonged overall survival (OS) versus standard chemotherapy in patients with previously treated locally advanced or metastatic urothelial carcinoma (mUC) previously receiving a programmed cell death protein 1/ligand 1 (PD-1/L1) inhibitor and platinum-based chemotherapy in the pivotal, phase 3 EV-301 clinical trial, supporting global approvals of enfortumab vedotin monotherapy. This bridging study was the first to evaluate enfortumab vedotin monotherapy in previously treated Chinese patients with locally advanced or mUC.
Methods: EV-203 was a multicenter, open-label, phase 2 study (NCT04995419) assessing efficacy, safety/tolerability, pharmacokinetics (PK), and immunogenicity of enfortumab vedotin in 40 Chinese patients (PK analysis set, n = 13) with previously treated locally advanced or mUC. Patients received enfortumab vedotin 1.25 mg/kg (Days 1, 8, and 15). Primary endpoints included confirmed objective response rate (ORR) by the independent review committee (IRC) and PK parameters of ADC, total antibody (TAb), and free monomethyl auristatin E (MMAE). Secondary endpoints included investigator-assessed confirmed ORR; investigator-/IRC-assessed duration of response (DOR), disease control rate (DCR), and progression-free survival (PFS); OS; immunogenicity; and safety/tolerability.
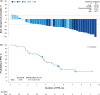
Results: As of May 13, 2022, the median follow-up was 6.5 months. Confirmed ORR was 37.5% (n/N = 15/40; 95% CI: 22.7%-54.2%) by IRC and 42.5% (n/N = 17/40; 95% CI: 27.0%-59.1%) by investigator assessment. By IRC, DCR was 72.5% (n = 29), median DOR was not reached, and median PFS was 4.7 months. Median OS was not reached. Endpoints assessed by investigators were consistent with IRC assessments. Two patients discontinued treatment for treatment-related adverse events. No new safety signals were identified. ADC, TAb, and free MMAE were characterized in Chinese patients and consistent with previously characterized populations. The incidence of positive antitherapeutic antibodies postbaseline was 0%.
Conclusion: Enfortumab vedotin demonstrated meaningful clinical activity with a manageable safety profile in Chinese patients with previously treated locally advanced or mUC.
Trial registration: ClinicalTrials.gov identifier: NCT04995419.
Keywords: antibody–drug conjugates; efficacy; monomethyl auristatin E; safety; urothelial carcinoma.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Siming Li, Yanxia Shi, Haiying Dong, Hongqian Guo, Yu Xie, Zhongquan Sun, and Xiaoping Zhang have no disclosures to report. Eric Kim is an employee of Pfizer Inc. Jun Zhang is an employee of Astellas Pharma Inc. Yue Li is an employee of Astellas Pharma Inc. Chenming Xu is an employee of Astellas Pharma Inc. Haishan Kadeerbai is an employee of Astellas Pharma Inc. Sue Lee was an employee of Astellas Pharma Inc. at the time of the study conduct and manuscript initiation. Seema Gorla is an employee of Astellas Pharma Inc. Jun Guo serves in consulting/advisory roles in Merck Sharp & Dohme, Roche, Bayer, Novartis, Simcere Pharmaceutical Group, Shanghai Junshi Biosciences, and Oriengene. Xinan Sheng reports a non‐profit consulting or advisory role in Pfizer, Astellas, AstraZeneca, MSD, Novartis, RemeGen, and Junshi Biosciences.
Figures
Similar articles
-
Enfortumab vedotin plus pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial cancer (EV-302): patient-reported outcomes from an open-label, randomised, controlled, phase 3 study.Lancet Oncol. 2025 Jun;26(6):795-805. doi: 10.1016/S1470-2045(25)00158-5. Lancet Oncol. 2025. PMID: 40449498 Clinical Trial.
-
Population Pharmacokinetic Modeling and Exposure-Response Analysis for the Antibody-Drug Conjugate Enfortumab Vedotin in Locally Advanced or Metastatic Urothelial Carcinoma.Clin Pharmacol Ther. 2024 Nov;116(5):1278-1288. doi: 10.1002/cpt.3383. Epub 2024 Jul 22. Clin Pharmacol Ther. 2024. PMID: 39039635
-
Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma.N Engl J Med. 2021 Mar 25;384(12):1125-1135. doi: 10.1056/NEJMoa2035807. Epub 2021 Feb 12. N Engl J Med. 2021. PMID: 33577729 Free PMC article. Clinical Trial.
-
Clinical Pharmacology of the Antibody-Drug Conjugate Enfortumab Vedotin in Advanced Urothelial Carcinoma and Other Malignant Solid Tumors.Clin Pharmacokinet. 2024 Apr;63(4):423-438. doi: 10.1007/s40262-024-01369-0. Epub 2024 Apr 12. Clin Pharmacokinet. 2024. PMID: 38609704 Free PMC article. Review.
-
Enfortumab vedotin and pembrolizumab: redefining the standard of care for previously untreated advanced urothelial cancer.Future Oncol. 2025 May;21(11):1333-1348. doi: 10.1080/14796694.2025.2482363. Epub 2025 Mar 25. Future Oncol. 2025. PMID: 40129250 Free PMC article. Review.
Cited by
-
Nectin-4 expression patterns and therapeutics in oncology.Cancer Lett. 2025 Jul 10;622:217681. doi: 10.1016/j.canlet.2025.217681. Epub 2025 Apr 8. Cancer Lett. 2025. PMID: 40209851 Review.
-
Research hotspots and frontier analysis of the novel immune checkpoint Nectin-4.Hum Vaccin Immunother. 2025 Dec;21(1):2504776. doi: 10.1080/21645515.2025.2504776. Epub 2025 May 17. Hum Vaccin Immunother. 2025. PMID: 40380880 Free PMC article. Review.
-
Multicenter study correlating molecular characteristics and clinical outcomes of cancer cases with patient-derived organoids.J Exp Clin Cancer Res. 2025 Jul 2;44(1):182. doi: 10.1186/s13046-025-03437-0. J Exp Clin Cancer Res. 2025. PMID: 40605011 Free PMC article.
References
-
- International Agency for Research on Cancer , “Data Visualization Tools for Exploring the Global Cancer Burden in 2022,” 2022. cited September 26, 2024, https://gco.iarc.fr/today/en.
-
- Bray F., Laversanne M., Sung H., et al., “Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 74, no. 3 (2024): 229–263. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

