Neutrophil swarming is crucial for limiting oral mucosal infection by Candida albicans
- PMID: 39530591
- PMCID: PMC11953071
- DOI: 10.1093/jleuko/qiae239
Neutrophil swarming is crucial for limiting oral mucosal infection by Candida albicans
Abstract
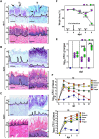
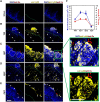
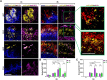
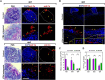
Oral mucosal colonization by Candida albicans is benign in healthy people but progresses to deeper infection, known as oropharyngeal candidiasis, that may become disseminated when combined with immunosuppression. Cortisone use and neutropenia are risk factors for invasive mucosal fungal infections; however, the mechanisms are poorly understood. Here, we identify in vivo neutrophil functional complexes known as swarms that are crucial for preventing C. albicans epithelial invasion. Anti-Ly6G antibody treatment impaired swarm formation and increased fungal infection depth, confirming the role of neutrophil swarms in limiting C. albicans invasion. Neutrophil swarm function could be disrupted by administration of resolvins, and required BLT1 (leukotriene B4 receptor 1) expression so that administration of a leukotriene synthesis inhibitor reduced neutrophil swarm size permitting C. albicans invasion beyond the basement membrane. Cortisone treatment similarly reduced neutrophil swarming behavior and BLT1 expression and delayed expression of epithelial cytokines and chemokines. Thus, swarm structures have an important function in preventing deep invasion by C. albicans within the oral mucosa and represent a mechanism for increased disease severity under immune deficient clinical settings.
Keywords: corticosteroids; epithelial invasion; neutrophil; oral candidiasis; swarming.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Leukocyte Biology.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures


Update of
-
Neutrophil swarms containing myeloid-derived suppressor cells are crucial for limiting oral mucosal infection by C. albicans.Res Sq [Preprint]. 2023 Oct 9:rs.3.rs-3346012. doi: 10.21203/rs.3.rs-3346012/v1. Res Sq. 2023. Update in: J Leukoc Biol. 2025 Mar 14;117(3):qiae239. doi: 10.1093/jleuko/qiae239. PMID: 37886517 Free PMC article. Updated. Preprint.
References
-
- Conti HR, Bruno VM, Childs EE, Daugherty S, Hunter JP, Mengesha BG, Saevig DL, Hendricks MR, Coleman BM, Brane L, et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe. 2016:20(5):606–617. 10.1016/j.chom.2016.10.001 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

