Antimicrobial Peptides: A Promising Alternative to Conventional Antimicrobials for Combating Polymicrobial Biofilms
- PMID: 39530703
- PMCID: PMC11714181
- DOI: 10.1002/advs.202410893
Antimicrobial Peptides: A Promising Alternative to Conventional Antimicrobials for Combating Polymicrobial Biofilms
Abstract
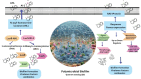
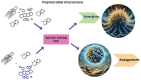
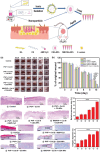
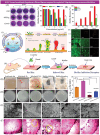
Polymicrobial biofilms adhere to surfaces and enhance pathogen resistance to conventional treatments, significantly contributing to chronic infections in the respiratory tract, oral cavity, chronic wounds, and on medical devices. This review examines antimicrobial peptides (AMPs) as a promising alternative to traditional antibiotics for treating biofilm-associated infections. AMPs, which can be produced as part of the innate immune response or synthesized therapeutically, have broad-spectrum antimicrobial activity, often disrupting microbial cell membranes and causing cell death. Many specifically target negatively charged bacterial membranes, unlike host cell membranes. Research shows AMPs effectively inhibit and disrupt polymicrobial biofilms and can enhance conventional antibiotics' efficacy. Preclinical and clinical research is advancing, with animal studies and clinical trials showing promise against multidrug-resistant bacteria and fungi. Numerous patents indicate increasing interest in AMPs. However, challenges such as peptide stability, potential cytotoxicity, and high production costs must be addressed. Ongoing research focuses on optimizing AMP structures, enhancing stability, and developing cost-effective production methods. In summary, AMPs offer a novel approach to combating biofilm-associated infections, with their unique mechanisms and synergistic potential with existing antibiotics positioning them as promising candidates for future treatments.
Keywords: antimicrobial alternatives; biofilms; drug discovery; polymicrobial interactions.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO , WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action, World Health Organization, Geneva: 2022, https://www.who.int/publications/i/item/9789240060241 (accessed: November 2024).
-
- WHO , WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance, World Health Organization, Geneva: 2024, https://www.who.int/publications/i/item/9789240093461 (accessed: November 2024).
-
- Reyes J., Komarow L., Chen L., Ge L., Hanson B. M., Cober E., Herc E., Alenazi T., Kaye K. S., Garcia‐Diaz J., Li L., Kanj S. S., Liu Z., Oñate J. M., Salata R. A., Marimuthu K., Gao H., Zong Z., Valderrama‐Beltrán S. L., Yu Y., Tambyah P., Weston G., Salcedo S., Abbo L. M., Xie Q., Ordoñez K., Wang M., Stryjewski M. E., Munita J. M., Paterson D. L., et al., Lancet Microbe 2023, 4, e159. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
