[Miller-Fisher/Guillain-Barré overlap syndrome following COVID-19 vaccination]
- PMID: 39530844
- PMCID: PMC12259147
- DOI: 10.5281/zenodo.10998949
[Miller-Fisher/Guillain-Barré overlap syndrome following COVID-19 vaccination]
Abstract
Background: Anti-GQ1B syndrome includes a group of diseases characterized by antibody-mediated polyneuropathy. Guillain Barre syndrome (GBS) and the Miller-Fisher syndrome (MFS) have been related to COVID-19 vaccine application.
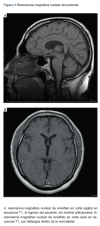
Clinic case: 48-year-old man, with history of Pfizer-BioNTech vaccination against COVID-19, 5 days prior to the symptoms, who assisted to the Emergency room with blurred vision and diplopia; adding dysarthria, facial diplegia and left upper limb weakness after 48 hours. In his first evaluation it was found ophthalmoplegia, facial diplegia, decreased gag reflex, weakness of thoracic limbs, bilateral trapezius muscle and areflexia. Serum studies and nuclear magnetic resonance of the brain were performed without alterations. It was complemented with IgG anti-ganglioside GQ1b antibodies with a positive result. Once the diagnosis was confirmed, treatment was started with immunoglobulin calculated at 2 g per kg for 5 days. The patient was discharged once the immunoglobulin was administered with evaluation at 2 months without ataxia, ophthalmoplegia, areflexia and weakness.
Conclusions: Following the documented reports of GBS and its variants secondary to vaccination, neurological side effects have been catalogued as being of great importance. Therefore, the reported case can be used as a point of reference to consider this clinical spectrum as a differential diagnosis in patients with post-vaccination neurological symptomatology.
Introducción: el síndrome anti-GQ1b reúne un grupo de enfermedades caracterizadas por un cuadro de polineuropatía mediada por anticuerpos. El síndrome de Guillain-Barré (SGB) y la variedad Miller-Fisher (SMF) se han relacionado con la aplicación de la vacuna contra COVID-19.
Caso clínico: hombre de 48 años, que se vacunó contra COVID-19 con Pfizer-BioNTech 5 días antes de los síntomas, el cual acudió a Urgencias con visión borrosa y diplopía; 48 horas después presentó disartria, diplejía facial y debilidad de miembro superior izquierdo. En la evaluación neurológica se encontró oftalmoplejía, diplejía facial, reflejo nauseoso disminuido, debilidad en miembros torácicos, músculo trapecio bilateral y arreflexia. Se realizaron estudios séricos y resonancia magnética nuclear de encéfalo sin alteraciones. Se complementó con anticuerpos IgG anti-gangliósido GQ1b con resultado positivo. Una vez que se confirmó el diagnóstico, se inició tratamiento con inmunoglobulina intravenosa (IGIV) calculada a 2 g por kg para 5 días. Finalizada la administración de IGIV, el paciente fue egresado con valoración a los 2 meses sin ataxia, oftalmoplejía, arreflexia ni debilidad.
Conclusiones: tras los reportes documentados de SGB y sus variantes secundarias a la vacunación, se han catalogado los efectos secundarios neurológicos de gran importancia. Por lo tanto, el caso reportado puede servir de referencia para considerar este espectro clínico como diagnóstico diferencial en pacientes con sintomatología neurológica posterior a la vacunación.
Keywords: COVID-19; COVID-19 Vaccines; Guillain-Barre Syndrome; Miller Fisher Syndrome.
Licencia CC 4.0 (BY-NC-ND) © 2024 Revista Médica del Instituto Mexicano del Seguro Social.
Conflict of interest statement
los autores han completado y enviado la forma traducida al español de la declaración de conflictos potenciales de interés del Comité Internacional de Editores de Revistas Médicas, y no fue reportado alguno que tuviera relación con este artículo.
Figures




Similar articles
-
Guillain-Barré syndrome after COVID-19 vaccination: A systematic review and analysis of case reports.Clin Neurol Neurosurg. 2024 Mar;238:108183. doi: 10.1016/j.clineuro.2024.108183. Epub 2024 Feb 20. Clin Neurol Neurosurg. 2024. PMID: 38401232
-
Efficacy of FcRn antagonist efgartigimod in the treatment of Miller-Fisher/Guillain-Barré overlap syndrome: Two case reports.J Neuroimmunol. 2025 Oct 15;407:578712. doi: 10.1016/j.jneuroim.2025.578712. Epub 2025 Aug 7. J Neuroimmunol. 2025. PMID: 40779911
-
Case Report: Therapeutic effect of efgartigimod in refractory anti-GQ1b antibody syndrome coexisting with myasthenia gravis.Front Immunol. 2025 Jun 10;16:1605985. doi: 10.3389/fimmu.2025.1605985. eCollection 2025. Front Immunol. 2025. PMID: 40557161 Free PMC article.
-
Miller-Fisher syndrome and Guillain-Barre syndrome overlap syndrome following inactivated COVID-19 vaccine: Case report and scope review.Hum Vaccin Immunother. 2022 Nov 30;18(6):2125753. doi: 10.1080/21645515.2022.2125753. Epub 2022 Oct 31. Hum Vaccin Immunother. 2022. PMID: 36315834 Free PMC article. Review.
-
Incidence and characteristics of Guillain-Barré syndrome in Taiwan before and during the COVID-19 pandemic: A 12-year single-center experience.J Chin Med Assoc. 2025 Jul 1;88(7):503-512. doi: 10.1097/JCMA.0000000000001251. Epub 2025 May 30. J Chin Med Assoc. 2025. PMID: 40442888
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

