The impact of aerobic and anaerobic exercise interventions on the management and outcomes of non-alcoholic fatty liver disease
- PMID: 39530904
- PMCID: PMC11629946
- DOI: 10.33549/physiolres.935244
The impact of aerobic and anaerobic exercise interventions on the management and outcomes of non-alcoholic fatty liver disease
Abstract
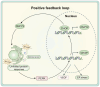
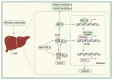
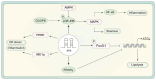
Non-alcoholic fatty liver disease (NAFLD) is a metabolic disorder that includes non-alcoholic hepatic steatosis without or with moderate inflammation and non-alcoholic steatohepatitis (NASH), characterized by necroinflammation and a more rapid progression of fibrosis. It is the primary pathological basis for hepatocellular carcinoma. With its prevalence escalating annually, NAFLD has emerged as a global health epidemic, presenting a significant hazard to public health worldwide. Existing studies have shown that physical activity and exercise training have a positive effect on NAFLD. However, the extent to which exercise improves NAFLD depends on the type, intensity, and duration. Therefore, the type of exercise that has the best effect on improving NAFLD remains to be explored. To date, the most valuable discussions involve aerobic and anaerobic exercise. Exercise intervenes in the pathological process of NAFLD by regulating physiological changes in cells through multiple signaling pathways. The review aims to summarize the signaling pathways affected by two different exercise types associated with the onset and progression of NAFLD. It provides a new basis for improving and managing NAFLD in clinical practice.
Conflict of interest statement
Figures
Similar articles
-
Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity.Korean J Intern Med. 2018 Jan;33(1):64-74. doi: 10.3904/kjim.2017.343. Epub 2017 Dec 6. Korean J Intern Med. 2018. PMID: 29202557 Free PMC article. Review.
-
The benefits of exercise for patients with non-alcoholic fatty liver disease.Expert Rev Gastroenterol Hepatol. 2015;9(10):1247-50. doi: 10.1586/17474124.2015.1075392. Epub 2015 Aug 4. Expert Rev Gastroenterol Hepatol. 2015. PMID: 26289101 Review.
-
Does aerobic exercise reduce NASH and liver fibrosis in patients with non-alcoholic fatty liver disease? A systematic literature review and meta-analysis.Front Endocrinol (Lausanne). 2022 Nov 3;13:1032164. doi: 10.3389/fendo.2022.1032164. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36407307 Free PMC article.
-
Non-alcoholic fatty liver disease: An overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions.Life Sci. 2021 Apr 15;271:119220. doi: 10.1016/j.lfs.2021.119220. Epub 2021 Feb 13. Life Sci. 2021. PMID: 33592199 Review.
-
Exerkines: Benign adaptation for exercise and benefits for non-alcoholic fatty liver disease.Biochem Biophys Res Commun. 2024 Sep 24;726:150305. doi: 10.1016/j.bbrc.2024.150305. Epub 2024 Jun 21. Biochem Biophys Res Commun. 2024. PMID: 38917635 Review.
Cited by
-
Protective effect of exercise on metabolic dysfunction‑associated fatty liver disease: Potential epigenetic mechanisms (Review).Int J Mol Med. 2025 Oct;56(4):146. doi: 10.3892/ijmm.2025.5587. Epub 2025 Jul 19. Int J Mol Med. 2025. PMID: 40682843 Free PMC article. Review.
References
-
- Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD) J Diabetes Res. 2020;2020:3920196. doi: 10.1155/2020/3920196. - DOI - PMC - PubMed
