A Phosphotriester-Masked Dideoxy-cGAMP Derivative as a Cell-Permeable STING Agonist
- PMID: 39531199
- PMCID: PMC11656140
- DOI: 10.1002/anie.202416353
A Phosphotriester-Masked Dideoxy-cGAMP Derivative as a Cell-Permeable STING Agonist
Abstract
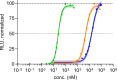
2',3'-Cyclic GMP-AMP (cGAMP) is a cyclic dinucleotide second messenger in which guanosine and adenosine are connected by one 3'-5' and one 2'-5' phosphodiester linkage. It is formed in the cytosol upon detection of pathogenic DNA by the enzyme guanosine-monophosphate-adenosine monophosphate synthase (cGAS). cGAMP subsequently binds to the adaptor protein stimulator of interferon genes (STING) to elicit an innate immune response leading to the production of type I interferons and cytokines. STING agonists are a highly promising avenue for an immuno-oncological anticancer therapy. A particular challenge with cyclic dinucleotide STING agonists are the two negative charges of the phosphodiester linkages, which strongly reduce the ability of such compounds to penetrate cell membranes. The development of cell-permeable STING agonists that can stimulate the immune system enhancing their anticancer potency is currently of utmost importance in the field. Herein, we report the development of a dideoxy derivative of cGAMP as a phosphotriester prodrug, where the negative charge of the phosphate backbone has been masked with a thioester. We found that this thioester-protected compound features a dramatic increase in its cellular potency that rises from EC50=5 μM to 25 nM. The new compound is envisioned to enable an efficient STING-agonist-based anticancer therapy.
Keywords: STING agonists; cyclic dinucleotides; immuno-oncology; prodrugs; thioesters.
© 2024 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

