12-Lipoxygenase inhibition delays onset of autoimmune diabetes in human gene replacement mice
- PMID: 39531315
- PMCID: PMC11665553
- DOI: 10.1172/jci.insight.185299
12-Lipoxygenase inhibition delays onset of autoimmune diabetes in human gene replacement mice
Abstract
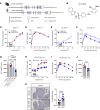
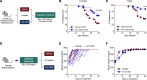
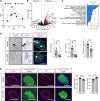
Type 1 diabetes (T1D) is characterized by the autoimmune destruction of insulin-producing β cells and involves an interplay between β cells and cells of the innate and adaptive immune systems. We investigated the therapeutic potential of targeting 12-lipoxygenase (12-LOX), an enzyme implicated in inflammatory pathways in β cells and macrophages, using a mouse model in which the endogenous mouse Alox15 gene is replaced by the human ALOX12 gene. Our finding demonstrated that VLX-1005, a potent 12-LOX inhibitor, effectively delayed the onset of autoimmune diabetes in human gene replacement non-obese diabetic mice. By spatial proteomics analysis, VLX-1005 treatment resulted in marked reductions in infiltrating T and B cells and macrophages, with accompanying increases in immune checkpoint molecule PD-L1, suggesting a shift toward an immunosuppressive microenvironment. RNA sequencing analysis of isolated islets and polarized proinflammatory macrophages revealed significant alteration of cytokine-responsive pathways and a reduction in IFN response after VLX-1005 treatment. Our studies demonstrated that the ALOX12 human replacement gene mouse provides a platform for the preclinical evaluation of LOX inhibitors and supports VLX-1005 as an inhibitor of human 12-LOX that engages the enzymatic target and alters the inflammatory phenotypes of islets and macrophages to promote the delay of autoimmune diabetes.
Keywords: Diabetes; Eicosanoids; Endocrinology; Islet cells; Therapeutics.
Conflict of interest statement
Figures


Update of
-
12-Lipoxygenase inhibition delays onset of autoimmune diabetes in human gene replacement mice.bioRxiv [Preprint]. 2024 Oct 29:2024.07.28.604986. doi: 10.1101/2024.07.28.604986. bioRxiv. 2024. Update in: JCI Insight. 2024 Dec 20;9(24):e185299. doi: 10.1172/jci.insight.185299. PMID: 39091839 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

