WD-repeat containing protein-61 regulates endometrial epithelial cell adhesion indicating an important role in receptivity
- PMID: 39531333
- PMCID: PMC11630898
- DOI: 10.1093/molehr/gaae039
WD-repeat containing protein-61 regulates endometrial epithelial cell adhesion indicating an important role in receptivity
Abstract
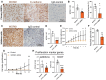
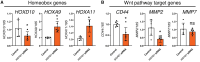
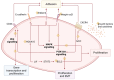
Endometrial receptivity is crucial for successful embryo implantation during early pregnancy. The human endometrium undergoes remodeling within each menstrual cycle to prepare or become receptive to an implanting blastocyst in the mid-secretory phase. However, the mechanisms behind these changes are not fully understood. Recently, using hormone-treated endometrial organoids to model receptivity, we identified that the transcriptional regulator WD-repeat-containing protein-61 (WDR61) was reduced in organoids derived from infertile women. In this study, we aimed to determine the role of WDR61 in endometrial receptivity. Here, we demonstrated that WDR61 immunolocalizes in the nuclei and cytosol of endometrial glandular epithelium, luminal epithelium, and stroma. The staining intensity of WDR61 was significantly higher during the receptive mid-secretory phase compared to the non-receptive proliferative phase in fertile women. In a functional experiment to model blastocyst adhesion to the endometrial epithelium, we found that adhesion of cytotrophoblast progenitor spheroids was blocked when siRNA was used to knockdown WDR61 in primary endometrial epithelial cells. Similarly, in Ishikawa cells (a receptive human endometrial epithelial cell line), siRNA knockdown of WDR61 significantly reduced the cell adhesive and proliferative capacities. qPCR revealed that WDR61 knockdown reduced expression of key genes involved in receptivity including HOXD10, MMP2, and CD44. Chromatin immunoprecipitation sequencing demonstrated that WDR61 directly targeted 2022 genes in Ishikawa cells, with functions including focal adhesion, intracellular signaling and epithelial-mesenchymal transition. Overall, these findings suggest that WDR61 promotes endometrial receptivity by modulating epithelial cell focal adhesions, proliferation, and epithelial-mesenchymal transition.
Keywords: Homeobox genes; WDR61; Wnt pathway; cell adhesion; chromatin immunoprecipitation sequencing; embryo implantation failure; endometrial epithelium; endometrial receptivity.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
This study was completed in the absence of any commercial or financial relationships. The authors have no conflict of interest to declare.
Figures



References
-
- Albayrak İG, Azhari F, Çolak EN, Balcı BK, Ülgen E, Sezerman U, Baştu E, Günel T.. Endometrial gene expression profiling of recurrent implantation failure after in vitro fertilization. Mol Biol Rep 2021;48:5075–5082. - PubMed
-
- Ali AFM, Cosemi E, Kamel S, Mohammed S, Elhefnawy M, Farid L, Shaker S.. ZAMZAM water stimulate BRAIN derived neurotrophic factor (BDNF) in uterine flushing of repeated implantation failure after (intracytoplasmic SPERUM injection)(ICSI). In: Thirteenth International Water Technology Conference. Citeseer, 2009. pp. 1549–1556.
-
- Azkargorta M, Bregón-Villahoz M, Escobes I, Ibáñez-Pérez J, Iloro I, Iglesias M, Diez-Zapirain M, Rabanal A, Prieto B, Moragues M-D et al In-depth proteomics and natural peptidomics analyses reveal antibacterial peptides in human endometrial fluid. J Proteomics 2020;216:103652. - PubMed
-
- Castelbaum AJ, Ying L, Somkuti SG, Sun J, Ilesanmi AO, Lessey BA.. Characterization of integrin expression in a well differentiated endometrial adenocarcinoma cell line (Ishikawa). J Clin Endocrinol Metab 1997;82:136–142. - PubMed
-
- Chen L, Nakai M, Belton RJ, Nowak RA.. Expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinases during mouse embryonic development. Reproduction 2007;133:405–414. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

