MAP3K1 mutations confer tumor immune heterogeneity in hormone receptor-positive HER2-negative breast cancer
- PMID: 39531335
- PMCID: PMC11735090
- DOI: 10.1172/JCI183656
MAP3K1 mutations confer tumor immune heterogeneity in hormone receptor-positive HER2-negative breast cancer
Abstract
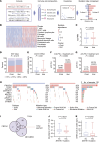
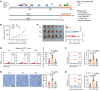
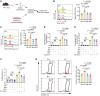
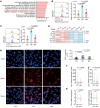
Treatment for hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) breast cancer, the most common type of breast cancer, has faced challenges such as endocrine therapy resistance and distant relapse. Immunotherapy has shown progress in treating triple-negative breast cancer, but immunological research on HR+/HER2- breast cancer is still in its early stages. Here, we performed a multi-omics analysis of a large cohort of patients with HR+/HER2- breast cancer (n = 351) and revealed that HR+/HER2- breast cancer possessed a highly heterogeneous tumor immune microenvironment. Notably, the immunological heterogeneity of HR+/HER2- breast cancer was related to mitogen-activated protein kinase kinase kinase 1 (MAP3K1) mutation and we validated experimentally that a MAP3K1 mutation could attenuate CD8+ T cell-mediated antitumor immunity. Mechanistically, MAP3K1 mutation suppressed MHC-I-mediated tumor antigen presentation through promoting the degradation of antigen peptide transporter 1/2 (TAP1/2) mRNA, thereby driving tumor immune escape. In preclinical models, the postbiotic tyramine could reverse the MAP3K1 mutation-induced MHC-I reduction, thereby augmenting the efficacy of immunotherapy. Collectively, our study identified the vital biomarker driving the immunological heterogeneity of HR+/HER2- breast cancer and elucidated the underlying molecular mechanisms, which provided the promise of tyramine as what we believe to be a novel therapeutic strategy to enhance the efficacy of immunotherapy.
Keywords: Cancer; Immunology; Immunotherapy; Oncology.
Figures
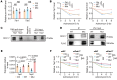
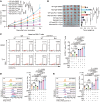
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

