Pembrolizumab 400 mg every 6 weeks as first-line therapy for advanced melanoma (KEYNOTE-555): Results from cohort B of an open-label, phase 1 study
- PMID: 39531423
- PMCID: PMC11556718
- DOI: 10.1371/journal.pone.0309778
Pembrolizumab 400 mg every 6 weeks as first-line therapy for advanced melanoma (KEYNOTE-555): Results from cohort B of an open-label, phase 1 study
Abstract
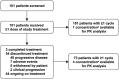
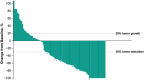
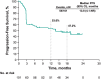
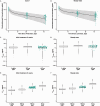
Intravenous pembrolizumab 400 mg every 6 weeks was approved across tumor types based on pharmacokinetic modeling, which showed exposures consistent with previous standard dosing of 200 mg or 2 mg/kg every 3 weeks, and early results of cohort B of the phase 1 KEYNOTE-555 study. Results after ≥1 year of potential follow-up for all patients in cohort B of KEYNOTE-555 are presented. Patients aged ≥18 years with previously untreated stage III/IV melanoma received pembrolizumab 400 mg every 6 weeks for ≤18 cycles. The primary endpoint was objective response rate per RECIST v1.1 by blinded independent central review. Secondary endpoints included duration of response, progression-free survival, pharmacokinetics, and safety. Overall, 101 patients received pembrolizumab. Median projected follow-up was 21.9 months (range, 17.0-25.7). The objective response rate was 50.5% (95% CI: 40.4-60.6; 19 complete responses, 32 partial responses). Median duration of response was not reached (NR; range, 2.4+ to 21.0+ months). Median progression-free survival was 13.8 months (95% CI: 4.1-NR). Observed pharmacokinetic exposures were consistent with model predictions for pembrolizumab 400 mg every 6 weeks and other approved and tested schedules (2 mg/kg or 200 mg every 3 weeks). Grade 3-4 treatment-related adverse events occurred in 13 patients (12.9%). No deaths were considered treatment related. These results support the pharmacokinetic modeling and demonstrate that the benefit-risk profile of pembrolizumab 400 mg Q6W is consistent with that of 200 mg or 2 mg/kg every 3 weeks. Clinically meaningful objective response rate and durable progression-free survival within the expected range for first-line pembrolizumab were observed. Clinical trial registry: ClinicalTrials.gov, NCT03665597.
Copyright: © 2024 Cohen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: BR reports honoraria, advisory/consultancy, and speakers bureau from MSD, Roche South Africa, and AstraZeneca South Africa; research grant/funding from Roche South Africa; and travel/accommodations/expenses from MSD. PR reports honoraria, advisory/consultancy, speakers bureau, research grant/funding, and travel/accommodations/expenses from Charlotte Maxeke Johannesburg Academic Hospital to their institution. AA reports honoraria, speakers bureau, research grant/funding, and travel/accommodations/expenses from Pierre-Fabre, Novartis, MSD, Bristol Myers Squibb, Roche, Merck, and Sanofi. MPB reports honoraria and advisory/consultancy from MSD, Bristol Myers Squibb, and Novartis to their institution; research grant/funding from MSD and BMS to their institution; and travel/accommodations/expenses from MSD. RMZ reports advisory/consultancy for AstraZeneca; speakers bureau for Limbic Oncology; and researching grant/funding to their institution from Bristol Myers Squibb and Roche. EMC reports consulting fees from Bristol Myers Squibb, Merck Serono, Amgen, Pierre-Fabre, Sanofi, and Roche; honoraria from Bristol Myers Squibb, Novartis, Sanofi, Merck Serono, and Pierre-Fabre; travel/accommodations/expenses from Bristol Myers Squibb, Novartis, Merck Serono, and Sun Pharmaceuticals; and participation on data safety monitoring board or advisory board for Bristol Myers Squibb. ML reports advisory/consultancy from Bristol Myers Squibb, MSD, Novartis, and Roche; speakers bureau from BMS and MSD; and travel/accommodations/expenses from Pierre-Fabre and Merck. JRA is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and stockholder of Merck & Co., Inc., Rahway, NJ, USA. LJ and DDA are a stockholders of Merck & Co., Inc., Rahway, NJ, USA. ML, OA, and EC are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, stockholders of Merck & Co., Inc., Rahway, NJ, USA, and hold patents/licensing/royalties/other intellectual property at Merck & Co., Inc., Rahway, NJ, USA. All remaining authors (GC, SWC, KME, BH, and CJ) have declared no conflicts of interest.
Figures
References
-
- KEYTRUDA® (pembrolizumab) injection, for intravenous use [prescribing information]. Merck & Co., Inc; Rahway, NJ: 2024.
-
- US Food and Drug Administration. FDA approves new dosing regimen for pembrolizumab Bethesda, MD: US Food and Drug Administration; 2022 [updated 04/28/2022; cited 2022 06/14/2022]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro....
-
- B.V. MSaD. KEYTRUDA 25 mg/mL concentrate for solution for infusion. (SPC). 2022. Merck Sharp and Dohme B.V.: The Netherlands; 2022. p. 116.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

