Unlocking the Potential of Push-Pull Pyridinic Photobases: Aggregation-Induced Excited-State Proton Transfer
- PMID: 39531467
- PMCID: PMC11739827
- DOI: 10.1002/chem.202403388
Unlocking the Potential of Push-Pull Pyridinic Photobases: Aggregation-Induced Excited-State Proton Transfer
Abstract
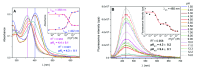
The pH effect on the photophysics of three push-pull compounds bearing dimethoxytriphenylamine (TPA-OMe) as electron donor and pyridine as electron acceptor, with different ortho-functionalization (-H, -Br, and -TPA-OMe), is assessed through steady-state and time-resolved spectroscopic techniques in DMSO/water mixed solutions and in water dispersions over a wide pH range. The enhanced intramolecular charge transfer upon protonation of the pyridinic ring leads to the acidochromic (from colorless to yellow) and acido(fluoro)chromic (from cyan to pink) behaviours of the investigated compounds. In dilute DMSO/buffer mixtures these molecules exhibited low pKa values (≤3.5) and extremely short singlet lifetimes. Nevertheless, it is by exploiting the aggregation phenomenon in aqueous environment that the practical use of these compounds largely expands: i) the basicity increases (pKa≈4.5) approaching the optimum values for pH-sensing in cancer cell recognition; ii) the fluorescence efficiencies are boosted due to Aggregation-Induced Emission (AIE), making these compounds appealing as fluorescent probes; iii) longer singlet lifetimes enable Excited-State Proton Transfer, paving the way for the application of these molecules as photobases (pKa*=9.1). The synergy of charge and proton transfers combined to the AIE behaviour in these pyridines allows tunable multi-responsive optical properties providing valuable information for the design of new light-emitting photobases.
Keywords: Aggregation-induced emission; Excited-state proton transfer; Photobases; Push-pull compounds; Ultrafast spectroscopy.
© 2024 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Prasad P. N., Williams D. J., Introduction to Nonlinear Optical Effects in Molecules and Polymers, Wiley, New York, 1991.
-
- Forrest S. R., Thompson M. E., Chem. Rev. 2007, 107, 923–925.
-
- Keerthi A., Sriramulu D., Liu Y., Yuan Timothy C. T., Wang Q., Valiyaveettil S., Dyes Pigm. 2013, 99, 787–797.
-
- Qin Y., Li G., Qi T., Huang H., Mater. Chem. Front. 2020, 4, 1554–1568.
-
- Biaggio I., Chem.-Eur. J. 2022, 28(6), e202103168. - PubMed
Grants and funding
- ECS00000041 - VITALITY/European Union - NextGenerationEU under the Italian Ministry of University and Research (MUR) National Innovation Ecosystem
- PRIN 2022 program, grant no. 2022RRFJC4/Italian Ministry of University and Research
- Project No. 01/3112/23/EMR-II/Council for Scientific and Industrial Research
- Project No. CRG/2022/000023 and STR/2022/000001/Science and Engineering Research Board (SERB)
LinkOut - more resources
Full Text Sources

