Hierarchical behavior control by a single class of interneurons
- PMID: 39531495
- PMCID: PMC11588054
- DOI: 10.1073/pnas.2410789121
Hierarchical behavior control by a single class of interneurons
Erratum in
-
Correction for Huo et al., Hierarchical behavior control by a single class of interneurons.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2424365121. doi: 10.1073/pnas.2424365121. Epub 2024 Dec 20. Proc Natl Acad Sci U S A. 2025. PMID: 39705319 Free PMC article. No abstract available.
Abstract
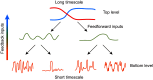
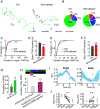
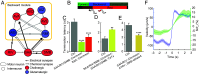
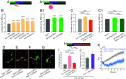
Animal behavior is organized into nested temporal patterns that span multiple timescales. This behavior hierarchy is believed to arise from a hierarchical neural architecture: Neurons near the top of the hierarchy are involved in planning, selecting, initiating, and maintaining motor programs, whereas those near the bottom of the hierarchy act in concert to produce fine spatiotemporal motor activity. In Caenorhabditis elegans, behavior on a long timescale emerges from ordered and flexible transitions between different behavioral states, such as forward, reversal, and turn. On a short timescale, different parts of the animal body coordinate fast rhythmic bending sequences to produce directional movements. Here, we show that Sublateral Anterior A (SAA), a class of interneurons that enable cross-communication between dorsal and ventral head motor neurons, play a dual role in shaping behavioral dynamics on different timescales. On a short timescale, SAA regulate and stabilize rhythmic bending activity during forward movements. On a long timescale, the same neurons suppress spontaneous reversals and facilitate reversal termination by inhibiting Ring Interneuron M (RIM), an integrating neuron that helps maintain a behavioral state. These results suggest that feedback from a lower-level cell assembly to a higher-level command center is essential for bridging behavioral dynamics at different levels.
Keywords: Caenorhabditis elegans; feedback inhibition; hierarchical behavior; organizing behavior timescales.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Tinbergen N., The Study of Instinct, The Study of Instinct (Clarendon Press/Oxford University Press, New York, NY, US, 1951), pp. xii, 237.
-
- Dawkins R., “Hierarchical organisation: A candidate principle for ethology” in Growing Points in Ethology, Bateson P. P. G., Hinde R. A., Eds. (Cambridge U Press, Oxford, England, 1976).
-
- Robertson R. J., Powers W. T., Introduction to Modern Psychology: The Control-Theory View (Control Systems Group, 1990), pp. ix, 238.
-
- Hölldobler B., Wilson E., The Ants (Springer, Berlin Heidelberg, 1998).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

