Efficient and selective kidney targeting by chemically modified carbohydrate conjugates
- PMID: 39532098
- PMCID: PMC11638880
- DOI: 10.1016/j.ymthe.2024.10.020
Efficient and selective kidney targeting by chemically modified carbohydrate conjugates
Abstract
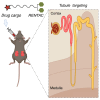
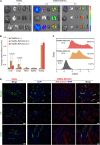
We investigated a renal tubule-targeting carbohydrate (RENTAC) that can selectively deliver small-molecule and nucleic acid analogs to the proximal convoluted tubules of the kidney following systemic delivery in mice. We comprehensively evaluated anti-miR-21-peptide nucleic acid-RENTAC, and fluorophore-RENTAC conjugates in cell culture and in vivo. We established that RENTAC conjugates showed megalin- and cubilin-dependent endocytic uptake in the immortalized kidney cell line. In vivo biodistribution studies confirmed the retention of RENTAC conjugates in the kidneys for several days compared with other organs. Immunofluorescence staining confirmed the selective distribution of the RENTAC conjugates in proximal convoluted tubules. We further demonstrated proximal convoluted tubule targeting features of RENTAC conjugates in a folic acid-induced kidney fibrosis mouse model. As a biological readout, we targeted miR-33 using antisense peptide nucleic acid (PNA) 33-RENTAC conjugates in the fibrotic kidney disease model. The targeted delivery of PNA 33-RENTAC resulted in slower fibrosis progression and decreased collagen deposition. We also confirmed that the RENTAC ligand did not exert any adverse reactions. Thus, we established that the RENTAC ligand can be used for broad clinical applications targeting the kidneys selectively.
Keywords: RENTAC; acetylated lactobionic acid; antisense oligonucleotide; cubilin; fibrosis; kidney delivery; megalin; miR-33; peptide nucleic acid; proximal convoluted tubules.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.B., V.K., and A.W. are inventors of patents related to kidney delivery assigned to the University of Connecticut.
Figures






References
-
- Crooke S.T., Baker B.F., Crooke R.M., Liang X.-h. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 2021;20:427–453. - PubMed
-
- Bennett C.F. Therapeutic antisense oligonucleotides are coming of age. Annu. Rev. Med. 2019;70:307–321. - PubMed
-
- Nair J.K., Willoughby J.L.S., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S., et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. - PubMed
-
- Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.-C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L., et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018;379:11–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

