Regulated GATA1 expression as a universal gene therapy for Diamond-Blackfan anemia
- PMID: 39532107
- PMCID: PMC11698655
- DOI: 10.1016/j.stem.2024.10.012
Regulated GATA1 expression as a universal gene therapy for Diamond-Blackfan anemia
Abstract
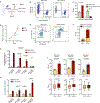
Gene therapy using hematopoietic stem and progenitor cells is altering the therapeutic landscape for patients with hematologic, immunologic, and metabolic disorders but has not yet been successfully developed for individuals with the bone marrow failure syndrome Diamond-Blackfan anemia (DBA). More than 30 mutations cause DBA through impaired ribosome function and lead to inefficient translation of the erythroid master regulator GATA1, providing a potential avenue for therapeutic intervention applicable to all patients with DBA, irrespective of the underlying genotype. Here, we report the development of a clinical-grade lentiviral gene therapy that achieves erythroid lineage-restricted expression of GATA1. We show that this vector is capable of augmenting erythropoiesis in DBA models and diverse patient samples without impacting hematopoietic stem cell function or demonstrating any signs of premalignant clonal expansion. These preclinical safety and efficacy data provide strong support for the first-in-human universal gene therapy trial for DBA through regulated GATA1 expression.
Keywords: Diamond-Blackfan anemia; GATA1; bone marrow failure; enhancer; erythropoiesis; gene therapy; hematopoiesis; hematopoietic stem cell; hypoplastic anemia; lentivirus.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests V.G.S. serves as an advisor to Ensoma, unrelated to the present work. R.A.V. and V.G.S. are named as inventors on patent PCT/US2020/036600 filed by Boston Children’s Hospital related to this approach.
Figures



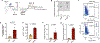


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

