Antifibrotic drug treatment of patients with idiopathic pulmonary fibrosis in Sweden: A registry-based observational study
- PMID: 39532288
- PMCID: PMC11558727
- DOI: 10.1177/14799731241299443
Antifibrotic drug treatment of patients with idiopathic pulmonary fibrosis in Sweden: A registry-based observational study
Abstract
Objectives: Idiopathic pulmonary fibrosis (IPF) is characterized by progressive fibrosis of the lung parenchyma, resulting in respiratory failure. This study analysed differences in patient characteristics and antifibrotic treatment strategies during the first years after IPF diagnosis.
Methods: Data from patients with IPF was extracted from the Swedish IPF registry. Patients were defined as treated (either as fully- or reduced treated) or non-treated with antifibrotic drugs. Differences in clinical parameters and side effects were defined.
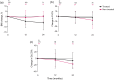
Results: Among 532 patients, 371 received treatment with antifibrotic drugs. Treated patients were younger, had worse lung function, higher body mass index (BMI), higher Gender-Age-Physiology stage, and were more often on oxygen treatment. Non-treated patients displayed a stable BMI, whereas patients treated with antifibrotics declined in BMI during follow-up. More than half (56%) of treated patients had reduced antifibrotic treatment. Sixty per cent reported side effects, with diarrhoea, nausea, and skin rash as the most common.
Conclusions: Patients prescribed antifibrotic treatment had more advanced disease compared to patients not prescribed antifibrotics. A considerable proportion of the patients had reduced treatment, probably due to more side effects in this group. This indicates that individuals starting treatment at IPF diagnosis are considered to be in greater need of antifibrotic drug treatment by the prescriber, compared to individuals with less severe disease.
Keywords: Idiopathic pulmonary fibrosis; body mass index; drug therapy; registries; side effects.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Speaker fees from Boehringer-Ingelheim and Roche.LC reports speaker fees from Boehringer-Ingelheim and Roche.DK declare that there is no conflict of interest.IP reports speaker fees from Boehringer-Ingelheim and Roche.JMM reports institutional grants and speaker fees from Boehringer-Ingelheim.MS reports research grants from Boehringer Ingelheim and Roche for pulmonary fibrosis research, payments for lecture and educational activities related to pulmonary fibrosis and participation on advisory boards related to pulmonary fibrosis.
Figures


References
-
- Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017; 389(10082): 1941–1952. - PubMed
-
- Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet 2022; 400(10354): 769–786. - PubMed
-
- Podolanczuk AJ, Thomson CC, Remy-Jardin M, et al. Idiopathic pulmonary fibrosis: state of the art for 2023. Eur Respir J 2023; 61(4): 2200957. - PubMed
-
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377(9779): 1760–1769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

