Generating Tooth Organoids Using Defined Bioorthogonally Cross-Linked Hydrogels
- PMID: 39532305
- PMCID: PMC11656705
- DOI: 10.1021/acsmacrolett.4c00520
Generating Tooth Organoids Using Defined Bioorthogonally Cross-Linked Hydrogels
Abstract
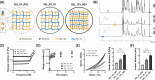
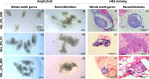
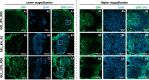
Generating teeth in vitro requires mimicking tooth developmental processes. Biomaterials are essential to support 3D tooth organoid formation, but their properties must be finely tuned to achieve the required biomimicry for tooth development. For the first time, we used bioorthogonally cross-linked hydrogels as defined 3D matrixes for tooth developmental engineering, and we highlighted how their properties play a pivotal role in enabling 3D tooth organoid formation in vitro. We prepared hydrogels by mixing gelatin precursors modified either with tetrazine (Tz) or norbornene (Nb) moieties. We tuned the hydrogel properties (E = 2-7 kPa; G' = 500-1500 Pa) by varying the gelatin concentration (8% vs 12% w/V) and stoichiometric ratio (Tz:Nb = 1 vs 0.5). We encapsulated dental epithelial-mesenchymal cell pellets in a library of hydrogels and identified a hydrogel formulation that enabled successful growth kinetics and morphogenesis of tooth germs, introducing a defined tunable platform for tooth organoid engineering and modeling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Bernabe E.; Marcenes W.; Hernandez C. R.; Bailey J.; Abreu L. G.; Alipour V.; Amini S.; Arabloo J.; Arefi Z.; Arora A.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. Journal of Dental Research 2020, 99 (4), 362–373. 10.1177/0022034520908533. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

