Short-term intraocular pressure changes after intravitreal aflibercept 2 mg, aflibercept 8 mg and faricimab: a prospective, comparative study
- PMID: 39532510
- PMCID: PMC12171477
- DOI: 10.1136/bjo-2024-326053
Short-term intraocular pressure changes after intravitreal aflibercept 2 mg, aflibercept 8 mg and faricimab: a prospective, comparative study
Abstract
Background/aims: Intravitreal injection (IVT) of anti-vascular endothelial growth factor agents is the standard of care for several retinal diseases but can cause intraocular pressure (IOP) elevations. This study investigates short-term postinjection IOP changes following aflibercept 8 mg and faricimab, compared with aflibercept 2 mg.
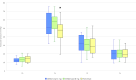
Methods: This observational, prospective study included 90 patients with age-related macular degeneration or diabetic macular oedema, divided into three groups, receiving aflibercept 2 mg, aflibercept 8 mg or faricimab. IOP was measured using an iCare IC200-tonometer preinjection (T0) and at 30 s (T1), 5 min (T2) and 15 min (T3) postinjection. Primary outcomes included IOP changes at the four time points within and between treatment groups. The incidence of transient visual loss requiring paracentesis was recorded.
Results: All groups experienced a significant IOP increase at T1, with mean IOP increase being 41.47±12.95 mm Hg for aflibercept 2 mg, 43.46±8.97 mm Hg for aflibercept 8 mg and 32.19±11.06 mm Hg for faricimab. By T2, IOP differences were not significant, and by T3, mean IOP returned within normal limits across all groups. Faricimab showed a smaller initial IOP spike than both aflibercept formulations, but this difference was not statistically significant at T2 and T3.
Conclusion: Transient IOP spikes are observed post-IVT of aflibercept 8 mg and faricimab, with similar trends to aflibercept 2 mg. The initial IOP elevation normalised within 15 min. Faricimab had a lower initial spike, but overall IOP profiles were comparable across different agents.
Keywords: Age-Related Macular Degeneration; Intraocular pressure; Retina; Vascular Endothelial Growth Factor.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AC provided consulting and/or speaker services for Santen. GG provided consulting and/or speaker services for Abbvie, Apellis, Bayer and Roche. MM provided consulting and/or speaker services for Abbvie, Apellis, Bayer, Novartis, Roche, and he is consultant and holds equity of Endogena.This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. All authors received an unrestricted grant from Bayer AG Switzerland, intended to support research activities at their hospital in a broader sense.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
