Harnessing synergistic effects of MMP-2 Inhibition and bFGF to simultaneously preserve and vascularize cardiac extracellular matrix after myocardial infarction
- PMID: 39532649
- PMCID: PMC11659021
- DOI: 10.1016/j.actbio.2024.10.050
Harnessing synergistic effects of MMP-2 Inhibition and bFGF to simultaneously preserve and vascularize cardiac extracellular matrix after myocardial infarction
Abstract
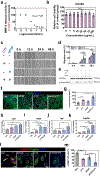
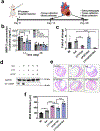
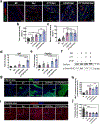
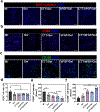
Myocardial infarction (MI) leads to cardiac extracellular matrix (ECM) degradation and fibrosis, reducing heart function. Consequently, simultaneously addressing ECM degradation and inhibiting cardiac fibrosis is essential for preserving heart function and mitigating adverse remodeling. However, the preserved ECM becomes unstable if not vascularized, as its structure and composition undergo changes over time. ECM vascularization is crucial to improve cardiac function. Presently, there is no clinically approved therapy that can simultaneously preserve and vascularize the ECM, and inhibit cardiac fibrosis. Our study develops a drug delivery system aiming to achieve these goals. It includes the peptide CTTHWGFTLC (CTT), a specific MMP-2 inhibitor, and basic fibroblast growth factor (bFGF), a potent factor with pro-angiogenic and anti-fibrotic properties. An injectable hydrogel serves as the carrier, featuring a rapid gelation that allows for the substantial retention of drugs. Additionally, the hydrogel has the capability to scavenge upregulated reactive oxygen species (ROS), thereby reducing tissue inflammation. Our findings indicate that CTT and bFGF synergistically enhance endothelial cell migration and tube formation while inhibiting the differentiation of fibroblasts into myofibroblasts. Upon delivery into hearts, the system significantly decreases MMP-2 level, promotes angiogenesis, attenuates cardiac fibrosis, and alleviates inflammation, resulting in a noteworthy cardiac function improvement. STATEMENT OF SIGNIFICANCE: 1) This work addresses key challenges in cardiac repair after myocardial infarction (MI), including extracellular matrix (ECM) degradation, vascularization, and fibrosis. 2) We combined an MMP-2/9 inhibitor (CTT) with bFGF to prevent ECM degradation, enhance vascularization, and inhibit fibrosis, providing a comprehensive strategy to improve cardiac function. 3) An injectable hydrogel was developed with rapid gelation and mechanical properties similar to heart tissue, ensuring efficient drug retention and reducing tissue stress. 4) The hydrogel enabled controlled, spatiotemporal release of CTT to dynamically reduce MMP-2/9 activity, and gradually released bFGF to promote angiogenesis and inhibit fibrosis.
Keywords: MMP-2 inhibitors; Myocardial infarction; ROS-scavenging; Vascularization; local delivery.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Rienks M, Papageorgiou A-P, Frangogiannis NG, Heymans S, Myocardial extracellular matrix: an ever-changing and diverse entity, Circ. Res 114 (5) (2014) 872–888. - PubMed
-
- Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling, Circulation 114 (10) (2006) 1020–1027. - PubMed
-
- Herzog E, Gu A, Kohmoto T, Burkhoff D, Hochman JS, Early activation of metalloproteinases after experimental myocardial infarction occurs in infarct and non-infarct zones, Cardiovasc. Pathol 7 (6) (1998) 307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

