Efficacy and safety of transcranial direct current stimulation (tDCS) in treatment of refractory epilepsy: an updated systematic review and meta-analysis of randomized sham-controlled trials
- PMID: 39532798
- PMCID: PMC11772517
- DOI: 10.1007/s10072-024-07866-1
Efficacy and safety of transcranial direct current stimulation (tDCS) in treatment of refractory epilepsy: an updated systematic review and meta-analysis of randomized sham-controlled trials
Abstract
Despite the currently available treatment, one-third of epilepsy patients continue to experience seizures. Transcranial direct current stimulation (tDCS) has emerged as a potential neuromodulation approach for the non-invasive treatment of refractory epilepsy. This study aims to provide a comprehensive investigation of the efficacy and safety of tDCS in patients with drug-resistant epilepsy. The following databases were searched from inception until June 2023; PubMed, Scopus, Embase, WOS, EBSCO, Cochrane Central, and Ovid MEDLINE. Pooled mean difference was calculated for change in seizure frequency (SF), and number of Interictal epileptiform discharges (IEDs) at different follow-up intervals. We included nine parallel randomized sham-controlled trials with a total of 267 patients. Active tDCS patients had a significantly lower SF per month at 4 and 8 weeks (MD = -4.06, 95% CI [-6.01 to -2.12], p < 0.0001), and (MD = -2.66, 95% CI [-5.09 to -0.23], p = 0.03), respectively. However, weekly SF showed no statistically significant results at 4 weeks of follow-up. The IEDs were observed to significantly decline at 2, 4, and 8 weeks of follow-up. The reported adverse events were mild including mild itching and erythematous rash that resolved spontaneously. In conclusion, tDCS significantly reduced monthly SF and the number of IEDs. Future large RCTs with standard clear informed parameters are still required.
Keywords: Interictal epileptiform discharges; Refractory epilepsy; Seizure frequency; Transcranial direct current stimulation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Conflict of interest: The authors declare no competing interests.
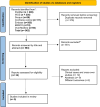
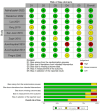
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

