Spatially integrated cortico-subcortical tracing data for analyses of rodent brain topographical organization
- PMID: 39532918
- PMCID: PMC11557934
- DOI: 10.1038/s41597-024-04060-y
Spatially integrated cortico-subcortical tracing data for analyses of rodent brain topographical organization
Abstract
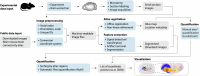
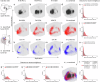
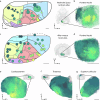
The cerebral cortex extends axonal projections to several subcortical brain regions, including the striatum, thalamus, superior colliculus, and pontine nuclei. Experimental tract-tracing studies have shown that these subcortical projections are topographically organized, reflecting the spatial organization of sensory surfaces and body parts. Several public collections of mouse- and rat- brain tract-tracing data are available, with the Allen mouse brain connectivity atlas being most prominent. There, a large body of image data can be inspected, but it is difficult to combine data from different experiments and compare spatial distribution patterns. To enable co-visualization and comparison of topographical organization in mouse brain cortico-subcortical projections across experiments, we represent axonal labelling data as point data in a common 3D brain atlas space. We here present a collection of point-cloud data representing spatial distribution of corticostriatal, corticothalamic, corticotectal, and corticopontine projections in mice and exemplify how these spatially integrated point data can be used as references for experimental investigations of topographic organization in transgenic mice, and for cross-species comparison with corticopontine projections in rats.
© 2024. The Author(s).
Conflict of interest statement
Figures



Similar articles
-
Three-Dimensional Histology Volume Reconstruction of Axonal Tract Tracing Data: Exploring Topographical Organization in Subcortical Projections from Rat Barrel Cortex.PLoS One. 2015 Sep 23;10(9):e0137571. doi: 10.1371/journal.pone.0137571. eCollection 2015. PLoS One. 2015. PMID: 26398192 Free PMC article.
-
The topography of corticopontine projections is controlled by postmitotic expression of the area-mapping gene Nr2f1.Development. 2022 Mar 1;149(5):dev200026. doi: 10.1242/dev.200026. Epub 2022 Mar 9. Development. 2022. PMID: 35262177 Free PMC article.
-
Analyzing Thalamocortical Tract-Tracing Experiments in a Common Reference Space.Neuroinformatics. 2024 Jan;22(1):23-43. doi: 10.1007/s12021-023-09644-4. Epub 2023 Oct 21. Neuroinformatics. 2024. PMID: 37864741 Free PMC article.
-
Clustered and laminar topographic patterns in rat cerebro-pontine pathways.Anat Embryol (Berl). 2003 Feb;206(3):149-62. doi: 10.1007/s00429-002-0272-7. Epub 2002 Oct 30. Anat Embryol (Berl). 2003. PMID: 12592566 Review.
-
The organization of corticothalamic projections: reciprocity versus parity.Brain Res Brain Res Rev. 1998 Dec;28(3):286-308. doi: 10.1016/s0165-0173(98)00017-4. Brain Res Brain Res Rev. 1998. PMID: 9858751 Review.
References
-
- Diamond, M. E., Petersen, R. S. & Harris, J. A. Learning through maps: functional significance of topographic organization in primary sensory cortex. J Neurobiol41, 64–68 (1999). - PubMed
-
- Chklovskii, D. B. & Koulakov, A. A. Maps in the brain: What can we learn from them? Annual Review of Neuroscience27, 369–392, 10.1146/annurev.neuro.27.070203.144226 (2004). - PubMed
-
- Kaas, J. H. Topographic maps are fundamental to sensory processing. Brain Res Bull44, 107–112, 10.1016/s0361-9230(97)00094-4 (1997). - PubMed
-
- Harding-Forrester, S. & Feldman, D. E. Somatosensory maps. 1 edn, Vol. 151 (Elsevier B.V., 2018). - PubMed
Publication types
MeSH terms
Grants and funding
- 785907/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945539/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 785907/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945539/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 269774/Norges Forskningsråd (Research Council of Norway)
LinkOut - more resources
Full Text Sources

