The effects of remdesivir on long-term symptoms in patients hospitalised for COVID-19: a pre-specified exploratory analysis
- PMID: 39533001
- PMCID: PMC11557865
- DOI: 10.1038/s43856-024-00650-4
The effects of remdesivir on long-term symptoms in patients hospitalised for COVID-19: a pre-specified exploratory analysis
Abstract
Background: There is an unmet need for treatment of long-term symptoms following COVID-19. Remdesivir is currently the only antiviral approved by the European Medicines Agency for hospitalised patients. Here, we report on the effect of remdesivir in addition to standard of care on long-term symptoms and quality of life in hospitalised patients with COVID-19 as part of the open-label randomised NOR-Solidarity trial (NCT04321616).
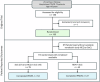
Methods: A total of 185 patients were included in the main trial, of which 118 (60%) were randomised to either remdesivir (n = 42; 36%) or a post-hoc defined control group composed of patients who received standard of care alone or standard of care with hydroxychloroquine (n = 76; 64%). Participants were given quality of life surveys to fill out to gauge their self-reported health over time (the COPD assessment test, the EQ-5D-5L and the RAND SF-36).
Results: Here we show that after three months, patients treated with remdesivir do not show significant improvements in stated health compared to those who were not. There are self-reported symptoms of fatigue [mean remdesivir group 2.6 (standard deviation 1.5) v control 2.1 (1.6), 95% confidence interval(CI) -1.17 to 0.15, p = 0.129], shortness of breath [3.0 (1.7) v 2.1 (1.8), 95% CI -1.53 to 0.16, p = 0.110] and coughing [1.8 (1.6) v 1.2 (1.5), 95% CI -1.3 to 0.33, p = 0.237] 3 months after randomisation assessed via the COPD Assessment Test.
Conclusions: Our findings indicate that treatment with remdesivir during hospitalisation does not provide any clinically relevant long-term benefit.
Plain language summary
Remdesivir is a medicine that is used to treat people with COVID-19. It has been found to help people get better faster, but we did not know whether it also relieved them of long-term symptoms such as persistent coughing, fatigue, or shortness of breath. To research this, we randomly assigned hospitalised patients with COVID-19 to either remdesivir on top of their normal care, or only normal care, with or without hydroxychloroquine (a drug later found to have no effect on COVID-19). We then compared participant’s symptoms after 3 months. Our results show that there is probably no benefit of using remdesivir during hospitalisation for long-term symptom relief.
© 2024. The Author(s).
Conflict of interest statement
Figures
Similar articles
-
Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses.Lancet. 2022 May 21;399(10339):1941-1953. doi: 10.1016/S0140-6736(22)00519-0. Epub 2022 May 2. Lancet. 2022. PMID: 35512728 Free PMC article.
-
Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial.Lancet Infect Dis. 2022 Feb;22(2):209-221. doi: 10.1016/S1473-3099(21)00485-0. Epub 2021 Sep 14. Lancet Infect Dis. 2022. PMID: 34534511 Free PMC article. Clinical Trial.
-
Remdesivir for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Aug 5;8(8):CD014962. doi: 10.1002/14651858.CD014962. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jan 25;1:CD014962. doi: 10.1002/14651858.CD014962.pub2. PMID: 34350582 Free PMC article. Updated.
-
Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial.Lancet Respir Med. 2021 Dec;9(12):1365-1376. doi: 10.1016/S2213-2600(21)00384-2. Epub 2021 Oct 18. Lancet Respir Med. 2021. PMID: 34672949 Free PMC article. Clinical Trial.
-
Remdesivir in Treatment of COVID-19: A Systematic Benefit-Risk Assessment.Drug Saf. 2020 Jul;43(7):645-656. doi: 10.1007/s40264-020-00952-1. Drug Saf. 2020. PMID: 32468196 Free PMC article.
References
-
- World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) [Internet]. Geneva, Switzerland: World Health Organisation (WHO); 2020; 30 January 2020. Available at https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting...-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
-
- World Health Organization Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses [published correction appears in Lancet. 2022 Oct 29;400(10362):1512]. Lancet.399, 1941–1953 (2022). - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical