Determinants of chemoselectivity in ubiquitination by the J2 family of ubiquitin-conjugating enzymes
- PMID: 39533056
- PMCID: PMC11649903
- DOI: 10.1038/s44318-024-00301-3
Determinants of chemoselectivity in ubiquitination by the J2 family of ubiquitin-conjugating enzymes
Abstract
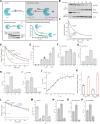
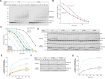
Ubiquitin-conjugating enzymes (E2) play a crucial role in the attachment of ubiquitin to proteins. Together with ubiquitin ligases (E3), they catalyze the transfer of ubiquitin (Ub) onto lysines with high chemoselectivity. A subfamily of E2s, including yeast Ubc6 and human Ube2J2, also mediates noncanonical modification of serines, but the structural determinants for this chemical versatility remain unknown. Using a combination of X-ray crystallography, molecular dynamics (MD) simulations, and reconstitution approaches, we have uncovered a two-layered mechanism that underlies this unique reactivity. A rearrangement of the Ubc6/Ube2J2 active site enhances the reactivity of the E2-Ub thioester, facilitating attack by weaker nucleophiles. Moreover, a conserved histidine in Ubc6/Ube2J2 activates a substrate serine by general base catalysis. Binding of RING-type E3 ligases further increases the serine selectivity inherent to Ubc6/Ube2J2, via an allosteric mechanism that requires specific positioning of the ubiquitin tail at the E2 active site. Our results elucidate how subtle structural modifications to the highly conserved E2 fold yield distinct enzymatic activity.
Keywords: ER-associated Protein Degradation; Noncanonical Ubiquitination; Posttranslational Modification; RING E3 Ligase; Ubiquitin-conjugating Enzyme.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures









References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2:19–25
-
- Ahel J, Fletcher A, Grabarczyk DB, Roitinger E, Deszcz L, Lehner A, Virdee S, Clausen T (2021) E3 ubiquitin ligase RNF213 employs a non-canonical zinc finger active site and is allosterically regulated by ATP. Preprint at bioRxiv 10.1101/2021.1105.1110.443411
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

