The potential for improving cardio-renal outcomes in chronic kidney disease with the aldosterone synthase inhibitor vicadrostat (BI 690517): a rationale for the EASi-KIDNEY trial
- PMID: 39533115
- PMCID: PMC12209857
- DOI: 10.1093/ndt/gfae263
The potential for improving cardio-renal outcomes in chronic kidney disease with the aldosterone synthase inhibitor vicadrostat (BI 690517): a rationale for the EASi-KIDNEY trial
Abstract
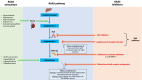
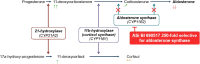
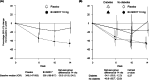
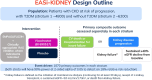
Patients with chronic kidney disease (CKD) are at risk of progressive loss of kidney function, heart failure, and cardiovascular death despite current proven therapies, including renin-angiotensin system inhibitors (RASi), sodium glucose co-transporter-2 inhibitors (SGLT2i), and statin-based regimens. RASi and SGLT2i reduce risk of CKD progression irrespective of primary cause of kidney disease, suggesting they target final common pathways. Targeting aldosterone overactivity with a nonsteroidal mineralocorticoid receptor antagonist (MRA) also reduces cardiorenal risk in patients with albuminuric diabetic kidney disease already treated with RASi. Together, these observations provide the rationale for trials to assess effects of inhibiting the aldosterone pathway in a broader range of patients with CKD, including those with non-diabetic causes of CKD or low albuminuria. Aldosterone synthase inhibitors (ASi) have emerged as an alternative to MRAs for aldosterone pathway inhibition. Phase II data from 586 patients with albuminuric CKD have shown that 10 mg of an ASi, vicadrostat (BI 690517), reduced urine albumin-to-creatinine ratio by ∼40% compared with placebo, with or without concurrent empagliflozin treatment. MRA and ASi increase risk of hyperkalaemia. Combining their use with an SGLT2i may mitigate some of this risk, improving tolerability, and allowing a wider range of patients to be treated (including those with higher levels of blood potassium than in previous trials). The EASi-KIDNEY (NCT06531824) double-blind placebo-controlled trial will test this approach by assessing the safety and cardiorenal efficacy of vicadrostat in combination with empagliflozin in ∼11 000 patients with CKD. It will be sufficiently large to assess effects in patients with and without diabetes separately.
Keywords: CKD; aldosterone; cardiovascular; clinical trial; heart failure.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
The EASi-KIDNEY trial was initiated, designed, and is being conducted, analysed, and reported by the University of Oxford with a Steering Committee of experts. This paper has not been published previously in whole or part. The Clinical Trial Service Unit and Epidemiological Studies Unit (Oxford, UK) has a staff policy of not accepting honorarium or other payments from the pharmaceutical industry, except for the reimbursement of costs to participate in scientific meetings (see
P.K.J., N.S., D.Z., R.J.S., N.A., R.A., K.J.M., E.S., C.W., M.J.L., C.B., R.H., and W.G.H. report grant funding paid to their institution (the University of Oxford) from Boehringer Ingelheim and Eli Lilly, and funding from the United Kingdom Medical Research Council (MRC) (to the Clinical Trial Service Unit and Epidemiological Studies Unit; reference no., MC_UU_00 017/3), the British Heart Foundation, National Institute for Health and Care Research Biomedical Research Council, and Health Data Research (UK). Additionally: P.K.J. reports institutional grant funding from Novartis. N.S. reports institutional grant funding from Novo Nordisk; and support to attend meetings from the Renal Research Institute. S.J.H., L.C., M.B., and S.V.S. are full-time employees of Boehringer Ingelheim International GmbH. M.J.L. additionally reports institutional grant funding from Novartis, Janssen, GV, Flu Lab, Schmidt Future, NHS England, Wellcome, Bill & Melinda Gates Foundation; research contract with Sanofi, Regeneron, Moderna, BioNTech, Apollo Therapeutics, Verve Therapeutics, and GSK; support to attend meetings from Boehringer Ingelheim; unpaid advisory role to European Society of Cardiology; and donation of treatment for clinical trials from Regeneron, Roche, Boehringer Ingelheim, and GSK. C.B. additionally reports institutional grant funding from NIHR HTA and Health Data Research UK; participation on a Data Safety Monitoring Board or Advisory Board related to Merck, NIHR HTA, the British Heart Foundation; and leadership roles as European Society of Cardiology Chair of Committee on Practice Guidelines and with NIHR HTA (Chair: ATTACK: Aspirin To Target Arterial Events in Chronic Kidney Disease; & DASH: Desmopressin for acute stroke due to haemorrhage). C.W. additionally reports institutional grant funding from Sanofi; consulting fees from Bayer, Boehringer Ingelheim, AstraZeneca, and Astellas; payment or honoraria for lectures from Bayer, Boehringer Ingelheim, AstraZeneca, Amgen, Sanofi, MSD, Fresenius Medical Care, CSL Vifor, Novartis, and Novo Nordisk. R.H. additionally reports institutional grant funding from Roche, GSK/Vir, and Combiphar; and participation on a Data Safety Monitoring Board or Advisory Board related to Lilly (no payments received). W.G.H. additionally reports a personal fellowship grant from Kidney Research UK. J.B.G. reports institutional grant funding from Merck, Roche, Boehringer Ingelheim, Lilly, Bluedrop; and consulting fees from AstraZeneca, NovoNordisk, Pfizer, Bayer, Anji, Boehringer Ingelheim, Valo, Lilly, Vertex, Mineralys. K.R.T. reports grant funding from the National Institutes of Health, NIH (NIDDK, NHLBI, NCATS, NIMHD), the Centers for Disease Control and Prevention (CDC), Travere, Bayer, and Doris Duke Foundation; consulting fees from Lilly, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Bayer, and ProKidney; honoraria from AstraZeneca, Novo Nordisk, and Bayer; support to attend meetings from Novo Nordisk; support for role on Data Safety Monitoring Board from AstraZeneca; unpaid roles on Data Safety Monitoring/Advisory Boards for NIDDK and George Clinical; paid roles on Data Safety Monitoring Board for AstraZeneca; and unpaid leadership roles as Chair, Diabetic Kidney Disease Collaborative Taskforce, American Society of Nephrology and Board of Directors, Kidney Disease Improving Global Outcomes, and Controversies Conference on Kidney Disease Prevention. P.R. reports institutional grant funding from Boehringer Ingelheim, AstraZeneca, Novo Nordisk, and Bayer; consulting fees from Bayer, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Sanofi, Gilead, and Novartis; and receipt of equipment, materials, drugs, medical writing, gifts or other services from Novo Nordisk, Bayer, and Lexicon. M.N. reports grant funding from Boehringer Ingelheim, Kyowa-Kirin, Daiichi-Sankyo, Astellas, Tanabe-Mitsubishi, JT, Chuga, Torii, Takeda, Bayer; consulting fees from Kyowa-Kirin, Astellas, Daiichi-Sankyo, Tanabe-Mitsubishi, JT, and Boehringer Ingelheim; and honoraria from Kyowa-Kirin, Astellas, AstraZeneca, GSK, Daiichi-Sankyo, Tanabe-Mitsubishi, Chugai, and Boehringer Ingelheim.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

