Stroke-Induced Renal Dysfunction: Underlying Mechanisms and Challenges of the Brain-Kidney Axis
- PMID: 39533116
- PMCID: PMC11557443
- DOI: 10.1111/cns.70114
Stroke-Induced Renal Dysfunction: Underlying Mechanisms and Challenges of the Brain-Kidney Axis
Abstract
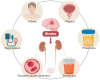
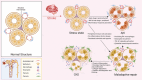
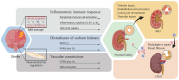
Stroke, a major neurological disorder and a leading cause of disability and death, often inflicts damage upon other organs, particularly the kidneys. While chronic kidney disease (CKD) has long been established as a significant risk factor for cerebrovascular disease, stroke can induce renal dysfunction, manifesting as acute kidney injury (AKI) or CKD. Mounting clinical and basic research evidence supports the existence of a bidirectional brain-kidney crosstalk following stroke, implicating specific mechanisms and pathways in stroke-related renal dysfunction. This review analyzes pertinent experimental studies, elucidating the underlying mechanisms of this cerebro-renal interaction following stroke. Additionally, we summarize the current landscape of clinical research investigating brain-kidney interplay and discuss potential challenges in the future. By enhancing our understanding of the scientific underpinnings of brain-kidney crosstalk, this review paves the way for improved treatment strategies and outcomes for stroke patients. Recognizing the intricate interplay between the brain and kidneys after stroke holds profound clinical implications.
Keywords: AKI; CKD; brain–kidney interaction; renal dysfunction; stroke.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Covic A., Schiller A., Mardare N. G., et al., “The Impact of Acute Kidney Injury on Short‐Term Survival in an Eastern European Population With Stroke,” Nephrology, Dialysis, Transplantation 23, no. 7 (2008): 2228–2234. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

