EGFR-TKIs or EGFR-TKIs combination treatments for untreated advanced EGFR-mutated NSCLC: a network meta-analysis
- PMID: 39533233
- PMCID: PMC11555867
- DOI: 10.1186/s12885-024-13168-8
EGFR-TKIs or EGFR-TKIs combination treatments for untreated advanced EGFR-mutated NSCLC: a network meta-analysis
Erratum in
-
Correction: EGFR-TKIs or EGFR-TKIs combination treatments for untreated advanced EGFR-mutated NSCLC: a network meta-analysis.BMC Cancer. 2025 Jan 28;25(1):166. doi: 10.1186/s12885-025-13577-3. BMC Cancer. 2025. PMID: 39875899 Free PMC article. No abstract available.
Abstract
Background: Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) and EGFR-TKI combination treatments have become the standard first-line treatments for EGFR-mutated non-small cell lung cancer (NSCLC) patients. However, the best option has yet to be determined. This study compares the efficacy and safety of various first-line EGFR-TKI monotherapies and combination treatments for advanced EGFR-mutated NSCLC.
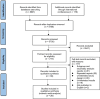
Methods: We searched PubMed, Embase, the Cochrane Central Register of Controlled Clinical Trials databases, and several international conferences to identify randomized controlled trials reporting on first-line EGFR-TKI treatments for patients with advanced EGFR-mutated NSCLC. The study quality was assessed using the revised tool for risk of bias in randomized trials. The efficacy and safety outcomes of the included treatments were compared by network meta-analysis based on a frequentist approach.
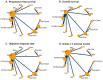
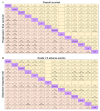
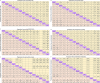
Results: We identified 26 trials (8,359 patients) investigating 14 treatment groups, including first, second, and third-generation EGFR-TKIs and their combination treatments. Osimertinib plus chemotherapy and lazertinib plus amivantamab showed the highest efficacy in improving progression-free survival. New third-generation EGFR-TKIs demonstrated comparable efficacy to osimertinib alone but did not surpass it. Subgroup analyses revealed slight variation in treatment efficacy based on mutation types and patient demographics. Combination treatments were associated with a higher incidence of adverse events.
Conclusion: These results reveal that osimertinib plus chemotherapy and lazertinib plus amivantamab are superior first-line options for patients with advanced EGFR-mutated NSCLC. However, these combinations are associated with higher adverse event rates.
Keywords: Amivantamab; EGFR-TKI; Lazertinib; Non-small cell lung cancer; Osimertinib.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624–39. - PubMed
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

