Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis refractory to biologic therapy: 2-year clinical and radiographic results from the open-label extension of the SELECT-AXIS 2 study
- PMID: 39533349
- PMCID: PMC11556075
- DOI: 10.1186/s13075-024-03412-8
Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis refractory to biologic therapy: 2-year clinical and radiographic results from the open-label extension of the SELECT-AXIS 2 study
Abstract
Background: The efficacy and safety of upadacitinib in patients with ankylosing spondylitis (AS) and inadequate response/intolerance to biologic disease-modifying antirheumatic drugs (bDMARD-IR) were evaluated through 1 year in the SELECT-AXIS 2 study. Here, we assess 2-year efficacy, safety, and imaging outcomes in SELECT-AXIS 2.
Methods: Patients who received continuous upadacitinib, and those who switched from placebo to upadacitinib at week 14, could enter the open-label extension (OLE). Efficacy endpoints included Assessment of SpondyloArthritis international Society (ASAS) and Axial Spondyloarthritis Disease Activity Score (ASDAS) responses, and changes from baseline in measures of disease activity, back pain, function, and quality of life. Radiographic progression was evaluated using the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS). As observed (AO) and AO with non-responder imputation (AO-NRI) analyses were used for binary endpoints; AO with mixed-effects model for repeated measures (AO-MMRM) for continuous endpoints; and AO-analysis of covariance for mSASSS. Treatment-emergent adverse events (TEAEs) in patients receiving ≥ 1 upadacitinib dose through week 104 are presented as events (E)/100 patient-years (PY). Subgroup analyses were performed by prior tumor necrosis factor/interleukin-17 inhibitor exposure and bDMARD lack of efficacy/intolerance.
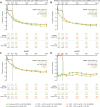
Results: Of 420 patients who entered the bDMARD-IR AS study, 409 entered the OLE, and 331 (continuous upadacitinib, n = 163; placebo to upadacitinib, n = 168) completed week 104. Improvements in efficacy measures were sustained through the OLE, with similar response rates between the continuous upadacitinib and placebo to upadacitinib groups at week 104. At week 104, 64.9% and 61.7% of patients, respectively, had achieved ASAS 40% response (AO-NRI). Mean changes from baseline were similar between the two groups at week 104 across measures (ASDAS: -2.1 and -2.0; total back pain: -4.9 and -4.6, respectively; AO-MMRM). Over 93.0% of patients showed no radiographic progression (mSASSS mean change from baseline < 2) at week 104. The overall TEAE rate was 165.2 E/100 PY, with low rates of major adverse cardiovascular and venous thromboembolic events (0.3 E/100 PY each).
Conclusions: Upadacitinib efficacy, including very low rates of radiographic progression, was demonstrated through 104 weeks in treatment-refractory patients with active AS. Treatment was well tolerated, with no newly identified safety signals.
Trial registration: NCT04169373.
Keywords: Ankylosing spondylitis; Biologic DMARD; Inadequate response; Open-label extension; Radiographic axial spondyloarthritis; Refractory; Upadacitinib.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Navarro-Compán V, Sepriano A, El-Zorkany B, van der Heijde D. Axial spondyloarthritis. Ann Rheum Dis. 2021;80(12):1511–21. - PubMed
-
- Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

