Prevalence of potential drug‒drug interactions and associated factors among elderly patients in Ethiopia: a systematic review and meta-analysis
- PMID: 39533381
- PMCID: PMC11559191
- DOI: 10.1186/s41256-024-00386-7
Prevalence of potential drug‒drug interactions and associated factors among elderly patients in Ethiopia: a systematic review and meta-analysis
Erratum in
-
Correction: Prevalence of potential drug‒drug interactions and associated factors among elderly patients in Ethiopia: a systematic review and meta-analysis.Glob Health Res Policy. 2024 Dec 30;9(1):54. doi: 10.1186/s41256-024-00402-w. Glob Health Res Policy. 2024. PMID: 39736690 Free PMC article. No abstract available.
Abstract
Background: The occurrence of potential drug‒drug interactions (pDDIs) is a serious global issue that affects all age groups, with the elderly population being the most vulnerable. This is due to their relatively high rates of comorbidity and polypharmacy, as well as physiological changes that can increase the potential for DDIs and the likelihood of adverse drug reactions. The aim of this study was to estimate the prevalence of pDDIs and associated factors among elderly patients in Ethiopia.
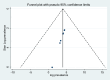
Methods: A comprehensive literature search using the preferred reporting items for systematic review and meta-analysis statement was conducted on HINARI, Science Direct, Embase, PubMed/MEDLINE, Google Scholar, and Research Gate. Data were extracted via a Microsoft Excel spreadsheet and analyzed via STATA version 11.0. Egger regression tests and funnel plot analysis were used to check publication bias, and the I2 statistic was used to evaluate statistical heterogeneity. Sensitivity and subgroup analyses were also conducted to identify potential causes of heterogeneity.
Results: Seven articles were analyzed, and a total of 1897 pDDIs were identified in 970 patients, resulting in an average of 1.97 DDIs per patient. The number of DDIs per patient ranged from 0.18 to 5.86. The overall prevalence of pDDIs among elderly patients was 50.69% (95% CI 18.77-82.63%). However, the prevalence of pDDIs ranged widely from 2.80 to 90.1%. When the severity of the interactions was considered, the prevalence of potential DDIs was found to be 28.74%, 70.68%, and 34.20% for major, moderate, and minor pDDIs, respectively. Polypharmacy and long hospital stays were identified as factors associated with pDDIs among elderly patients in Ethiopia.
Conclusions: The overall prevalence of pDDIs among elderly patients was high, with a wide range of prevalence rates. Moderate-severity interactions were the most prevalent. Polypharmacy and long hospital stays were identified as factors associated with pDDIs among elderly patients. The study suggests that DDIs identification database itself could have modified the DDIs prevalence rate. As a result, a single DDIs identification database needs to be authorized; otherwise, clinical knowledge should be taken into account when interpreting the information obtained.
Keywords: Associated factors; Elderly; Ethiopia; Potential drug‒drug interaction; Prevalence.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Hadjibabaie M, Badri S, Ataei S, Moslehi AH, Karimzadeh I, Ghavamzadeh A. Potential drug–drug interactions at a referral hematology–oncology ward in Iran: a cross-sectional study. Cancer Chemother Pharmacol. 2013;71:1619–27. - PubMed
-
- Marusic S, Bacic-Vrca V, Obreli Neto PR, Franic M, Erdeljic V, Gojo-Tomic N. Actual drug–drug interactions in elderly patients discharged from internal medicine clinic: a prospective observational study. Eur J Clin Pharmacol. 2013;69:1717–24. - PubMed
-
- Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Expert Opin Drug Saf. 2012;11(1):83–94. - PubMed
-
- Varma MV, Pang KS, Isoherranen N, Zhao P. Dealing with the complex drug–drug interactions: towards mechanistic models. Biopharm Drug Dispos. 2015;36(2):71–92. - PubMed
-
- Bjornsson T. Pharmaceutical research and manufacturers of America (PhRMA) drug metabolism/clinical pharmacology technical working group; FDA center for drug evaluation and research (CDER). The conduct of in vitro and in vivo drug-drug interaction studies: a pharmaceutical research and manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31(7):815–32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials