Bacteriocin Microcin J25's antibacterial infection effects and novel non-microbial regulatory mechanisms: differential regulation of dopaminergic receptors
- PMID: 39533384
- PMCID: PMC11559059
- DOI: 10.1186/s40104-024-01115-3
Bacteriocin Microcin J25's antibacterial infection effects and novel non-microbial regulatory mechanisms: differential regulation of dopaminergic receptors
Abstract
Background: The antibacterial and immunomodulatory activities of bacteriocins make them attractive targets for development as anti-infective drugs. Although the importance of the enteric nervous system (ENS) in the struggle against infections of the intestine has been demonstrated, whether it is involved in bacteriocins anti-infective mechanisms is poorly defined.
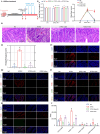
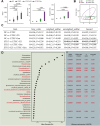
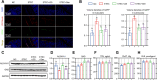
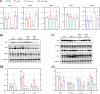
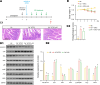
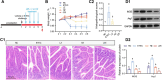
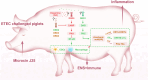
Results: Here, we demonstrated that the bacteriocin Microcin J25 (J25) significantly alleviated diarrhea and intestinal inflammation in piglets caused by enterotoxigenic Escherichia coli (ETEC) infection. Mechanistically, macrophage levels were significantly downregulated after J25 treatment, and this was replicated in a mouse model. Omics analysis and validation screening revealed that J25 treatment induced significant changes in the dopaminergic neuron pathway, but little change in microbial structure. The alleviation of inflammation may occur by down-regulating dopamine receptor (DR) D1 and the downstream DAG-PKC pathway, thus inhibiting arachidonic acid decomposition, and the inhibition of macrophages may occur through the up-regulation of DRD5 and the downstream cAMP-PKA pathway, thus inhibiting NF-κB.
Conclusions: Our studies' findings provide insight into the changes and possible roles of the ENS in J25 treatment of ETEC infection, providing a more sophisticated foundational understanding for developing the application potential of J25.
Keywords: Arachidonic acid; Dopaminergic receptors; Enteric nervous system; Macrophage; Microbiota; Microcins.
© 2024. The Author(s).
Conflict of interest statement
Figures
Similar articles
-
Lasso Peptide Microcin J25 Effectively Enhances Gut Barrier Function and Modulates Inflammatory Response in an Enterotoxigenic Escherichia coli-Challenged Mouse Model.Int J Mol Sci. 2020 Sep 5;21(18):6500. doi: 10.3390/ijms21186500. Int J Mol Sci. 2020. PMID: 32899529 Free PMC article.
-
Protective Ability of Biogenic Antimicrobial Peptide Microcin J25 Against Enterotoxigenic Escherichia Coli-Induced Intestinal Epithelial Dysfunction and Inflammatory Responses IPEC-J2 Cells.Front Cell Infect Microbiol. 2018 Jul 13;8:242. doi: 10.3389/fcimb.2018.00242. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30057893 Free PMC article.
-
Biosynthetic Microcin J25 Exerts Strong Antibacterial, Anti-Inflammatory Activities, Low Cytotoxicity Without Increasing Drug-Resistance to Bacteria Target.Front Immunol. 2022 Feb 18;13:811378. doi: 10.3389/fimmu.2022.811378. eCollection 2022. Front Immunol. 2022. PMID: 35250983 Free PMC article.
-
Focus on modified microcins: structural features and mechanisms of action.Biochimie. 2002 May-Jun;84(5-6):511-9. doi: 10.1016/s0300-9084(02)01411-6. Biochimie. 2002. PMID: 12423795 Review.
-
Low-molecular-weight post-translationally modified microcins.Mol Microbiol. 2007 Sep;65(6):1380-94. doi: 10.1111/j.1365-2958.2007.05874.x. Epub 2007 Aug 17. Mol Microbiol. 2007. PMID: 17711420 Review.
References
-
- Bali V, Panesar PS, Bera MB, Kennedy JF. Bacteriocins: recent trends and potential applications. Crit Rev Food Sci Nutr. 2016;56(5):817–34. 10.1080/10408398.2012.729231. - PubMed
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–95. 10.1038/415389a. - PubMed
-
- Fjell CD, Hiss JA, Hancock RE, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2011;11(1):37–51. 10.1038/nrd3591. - PubMed
-
- Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16(5):321–34. 10.1038/nri.2016.29. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

