Prevalence of comorbid autoimmune diseases and antibodies in newly diagnosed multiple sclerosis patients
- PMID: 39533435
- PMCID: PMC11556020
- DOI: 10.1186/s42466-024-00351-2
Prevalence of comorbid autoimmune diseases and antibodies in newly diagnosed multiple sclerosis patients
Abstract
Background: Diagnosing multiple sclerosis (MS) is challenging due to diverse symptoms and the absence of specific biomarkers. Concurrent autoimmune diseases (AID) or non-specific antibodies further complicate diagnosis, progression monitoring, and management. Data on AID prevalence in MS patients are sparse. This study aims to identify concurrent AIDs alongside MS.
Methods: In this retrospective single-center study, we analyzed patient records at our university hospital from 2010 to 2017, focusing on cases suspected of inflammatory demyelinating disease. The 2017 McDonald criteria were applied. Additionally, we measured neurofilament light (NfL) levels from available CSF samples in our biobank.
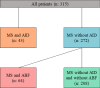
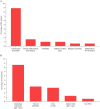
Results: We identified a total of 315 patients, of whom 66% were women. In total, 13.7% of all patients had concurrent AID, while 20.3% had isolated antibody findings without AID. The most common AID was autoimmune thyroiditis (8.9%), followed by chronic inflammatory skin diseases (1.6%), arthritis (1%), type 1 diabetes (1%), Sjögren's syndrome (0.6%), and inflammatory bowel diseases (0.6%). Cardiolipin antibodies were the most frequent isolated antibody finding (8.6%). Our data showed that, from the perspective of the initial demyelinating event, neither comorbid AID nor isolated antibodies significantly influenced relapses or MS progression over a median follow-up of 9 months. Standard CSF parameters and NfL levels were similar between the groups at the time of MS diagnosis.
Conclusion: Our study shows that AIDs, particularly autoimmune thyroiditis, frequently occur at the onset of MS. The proportion of AIDs commonly treated with immunomodulatory therapy in our cohort was similar to that observed in the general population. Comorbid AID did not affect NfL levels, indicating similar disease activity. Future research should explore new AID emergence during the course of MS, especially considering the increased incidence of rheumatic diseases later in life.
Keywords: Autoimmune diseases; Comorbidity; Multiple sclerosis; Neurofilament light; Prevalence.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Wang, L., Wang, F.-S., & Gershwin, M. E. (2015). Human autoimmune diseases: A comprehensive update. Journal of Internal Medicine,278(4), 369–395. 10.1111/joim.12395 - PubMed
-
- Walton, C., King, R., Rechtman, L., Kaye, W., Leray, E., Marrie, R. A., Robertson, N., La Rocca, N., Uitdehaag, B., van der Mei, I., Wallin, M., Helme, A., Napier, C. A., Rijke, N., & Baneke, P. (2020). Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Multiple Sclerosis Journal,26(14), 1816–1821. 10.1177/1352458520970841 - PMC - PubMed
-
- McDonald, W. I., Compston, A., Edan, G., Goodkin, D., Hartung, H.-P., Lublin, F. D., McFarland, H. F., Paty, D. W., Polman, C. H., Reingold, S. C., Sandberg-Wollheim, M., Sibley, W., Thompson, A., Van Den Noort, S., Weinshenker, B. Y., & Wolinsky, J. S. (2001). Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology,50(1), 121–127. 10.1002/ana.1032 - PubMed
-
- Polman, C. H., Reingold, S. C., Edan, G., Filippi, M., Hartung, H.-P., Kappos, L., Lublin, F. D., Metz, L. M., McFarland, H. F., O’Connor, P. W., Sandberg-Wollheim, M., Thompson, A. J., Weinshenker, B. G., & Wolinsky, J. S. (2005). Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Annals of Neurology,58(6), 840–846. 10.1002/ana.20703 - PubMed
LinkOut - more resources
Full Text Sources
