Low Molecular Weight Alginate Oligosaccharides as Alternatives to PEG for Enhancement of the Diffusion of Cationic Nanoparticles Through Cystic Fibrosis Mucus
- PMID: 39533498
- PMCID: PMC11694082
- DOI: 10.1002/adhm.202400510
Low Molecular Weight Alginate Oligosaccharides as Alternatives to PEG for Enhancement of the Diffusion of Cationic Nanoparticles Through Cystic Fibrosis Mucus
Abstract
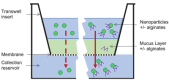
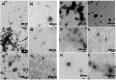
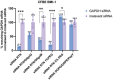
Airway mucus is a major barrier to the delivery of lipid-based nanoparticles in chronic airway diseases such as cystic fibrosis (CF). Receptor-Targeted Nanocomplexes (RTN), comprise mixtures of cationic lipids and bifunctional peptides with receptor-targeting and nucleic acid packaging properties. The aim of this study is to improve the mucus-penetrating properties of cationic siRNA and mRNA RTNs by combining them with low molecular weight alginate oligosaccharides, OligoG and OligoM. Cationic RTNs formulated with either alginate become strongly anionic, while PEGylated messenger RNA (mRNA) and short interfering RNA (siRNA) RTNs remain cationic. Both alginates enhance mucus diffusion rates of cationic siRNA and mRNA RTNs in a static mucus barrier diffusion model, with OligoG particularly effective. PEGylation also enhance mucus diffusion rates of siRNA RTNs but not mRNA RTNs. Electron microscopy shows that RTNs remained intact after mucosal transit. The transfection efficiency of OligoM-coated mRNA RTNs is better than those coated with OligoG or PEG, and similar to cationic RTNs. In siRNA RTN transfections, OligoM is better than OligoG although 1% PEG is slightly better than both. The combination of cationic RTNs and alginate oligosaccharides represents a promising alternative to PEGylation for epithelial delivery of genetic therapies across the mucus barrier while retaining transfection efficiency.
Keywords: cystic fibrosis; mRNA; mucus penetration; nanoparticles; siRNA.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
O. Alexander H. Åstrand and Philip D. Rye are employees and shareholders at AlgiPharma. Anne Tøndervik is an employee at SINTEF.
Figures





Similar articles
-
Hybrid Lipid/Polymer Nanoparticles to Tackle the Cystic Fibrosis Mucus Barrier in siRNA Delivery to the Lungs: Does PEGylation Make the Difference?ACS Appl Mater Interfaces. 2022 Feb 16;14(6):7565-7578. doi: 10.1021/acsami.1c14975. Epub 2022 Feb 2. ACS Appl Mater Interfaces. 2022. PMID: 35107987 Free PMC article.
-
Tumor-specific gene transfer with receptor-mediated nanocomplexes modified by polyethylene glycol shielding and endosomally cleavable lipid and peptide linkers.FASEB J. 2010 Jul;24(7):2301-13. doi: 10.1096/fj.09-144220. Epub 2010 Mar 4. FASEB J. 2010. PMID: 20203088
-
OligoG CF-5/20 normalizes cystic fibrosis mucus by chelating calcium.Clin Exp Pharmacol Physiol. 2017 Jun;44(6):639-647. doi: 10.1111/1440-1681.12744. Clin Exp Pharmacol Physiol. 2017. PMID: 28261854
-
PEGylation for enhancing nanoparticle diffusion in mucus.Adv Drug Deliv Rev. 2018 Jan 15;124:125-139. doi: 10.1016/j.addr.2017.08.010. Epub 2017 Sep 4. Adv Drug Deliv Rev. 2018. PMID: 28882703 Review.
-
Beyond PEGylation: Alternative surface-modification of nanoparticles with mucus-inert biomaterials.Adv Drug Deliv Rev. 2018 Jan 15;124:140-149. doi: 10.1016/j.addr.2017.07.015. Epub 2017 Jul 20. Adv Drug Deliv Rev. 2018. PMID: 28736302 Review.
References
-
- Nafee N., Forier K., Braeckmans K., Schneider M., Eur. J. Pharm. Biopharm. 2018, 124, 125. - PubMed
-
- a) Garbuzenko O. B., Kbah N., Kuzmov A., Pogrebnyak N., Pozharov V., Minko T., J. Control Release 2019, 296, 225; - PMC - PubMed
- b) Mastorakos P., da Silva A. L., Chisholm J., Song E., Choi W. K., Boyle M. P., Morales M. M., Hanes J., Suk J. S., Proc. Natl. Acad. Sci., U. S. A. 2015, 112, 8720; - PMC - PubMed
- c) Mottais A., Le Gall T., Sibiril Y., Ravel J., Laurent V., d'Arbonneau F., Montier T., Biosci. Rep. 2017, 37, BSR20160618; - PMC - PubMed
- d) Sardo C., Di Domenico E. G., Porsio B., De Rocco D., Santucci R., Ascenzioni F., Giammona G., Cavallaro G., Int. J. Pharm. 2019, 563, 347; - PubMed
- e) Torge A., Wagner S., Chaves P. S., Oliveira E. G., Guterres S. S., Pohlmann A. R., Titz A., Schneider M., Beck R. C. R., Int. J. Pharm. 2017, 527, 92. - PubMed
-
- Stern M., Caplen N. J., Browning J. E., Griesenbach U., Sorgi F., Huang L., Gruenert D. C., Marriot C., Crystal R. G., Geddes D. M., Alton E. W., Gene Ther. 1998, 5, 91. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

