The impact of Tanreqing injection on mucus hypersecretion and cough in bronchiectasis: A meta-analysis of randomized controlled trials
- PMID: 39533566
- PMCID: PMC11557049
- DOI: 10.1097/MD.0000000000040465
The impact of Tanreqing injection on mucus hypersecretion and cough in bronchiectasis: A meta-analysis of randomized controlled trials
Abstract
Background: Bronchiectasis clinically manifests airway mucus hypersecretion as mucopurulent sputum production and chronic cough. In the past decade, Tanreqing injection (TRQ) has been often used in clinical practice as an add-on treatment for bronchiectasis in China. Several in vivo studies have indicated that TRQ is effective in improving sputum expectoration and cough in acute exacerbation of bronchiectasis but results of individual studies are inconsistent. Therefore, systematically and critically evaluating the effectiveness and safety of TRQ on mucus hypersecretion and cough in bronchiectasis is necessary.
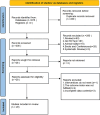
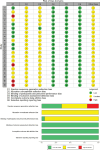
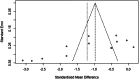
Methods: Randomized controlled trials examining the treatment of bronchiectasis with TRQ were systematically searched from databases including PubMed, Cochrane Library, Embase, Web of Science, Chinese National Knowledge Infrastructure, Vip Information Database, Wanfang data, and Chinese Biomedical Literature Database, based on a preregistered protocol and adhering to Cochrane methods. Pertinent data were taken out from the included studies and a methodological quality assessment was done. R language (version 4.4.1) was used to perform the meta-analysis.
Results: Twenty randomized controlled trials involving 1544 patients were analyzed. The results demonstrated that TRQ significantly improved mucus hypersecretion, shortened the duration of cough and phlegm, reduced symptom scores, and enhanced both forced expiratory volume in 1 second and forced vital capacity. Additionally, TRQ effectively lowered inflammatory markers, including C-reactive protein, procalcitonin, white blood cell count, neutrophil count, interleukin-6, and tumor necrosis factor-alpha. Moreover, TRQ increased the partial pressure of oxygen and decreased carbon dioxide pressure.
Conclusion: The findings suggest that TRQ positively impacts mucus hypersecretion and mucociliary clearance, leading to improvements in sputum production and cough during bronchiectasis exacerbations, without increasing the risk of adverse effects. TRQ may be considered a viable option for managing bronchiectasis and could serve as a novel mucus-modifying agent.
Copyright © 2024 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
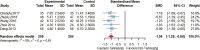

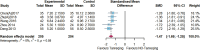
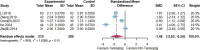

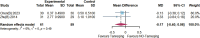

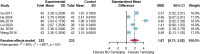



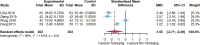
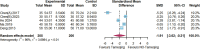

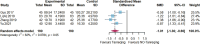
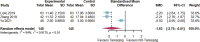


Similar articles
-
Systems pharmacology-based study of Tanreqing injection in airway mucus hypersecretion.J Ethnopharmacol. 2020 Mar 1;249:112425. doi: 10.1016/j.jep.2019.112425. Epub 2019 Nov 22. J Ethnopharmacol. 2020. PMID: 31765763
-
Efficacy and safety of Tanreqing injection for cough caused by acute trachea-bronchitis disease: A systematic review and meta-analysis of randomized controlled trials.J Ethnopharmacol. 2024 Mar 1;321:117429. doi: 10.1016/j.jep.2023.117429. Epub 2023 Nov 23. J Ethnopharmacol. 2024. PMID: 38007165
-
[A study of the mechanism of Qingre Huatan therapy in treatment of acute exacerbation of chronic obstructive pulmonary disease by improving airway inflammation and mucus hypersecretion].Zhong Xi Yi Jie He Xue Bao. 2008 Aug;6(8):799-805. doi: 10.3736/jcim20080806. Zhong Xi Yi Jie He Xue Bao. 2008. PMID: 18664347 Clinical Trial. Chinese.
-
Chronic cough and sputum production are associated with worse clinical outcomes in stable asthma.Respir Med. 2013 Oct;107(10):1501-8. doi: 10.1016/j.rmed.2013.07.017. Epub 2013 Aug 6. Respir Med. 2013. PMID: 23927851
-
Clinical Efficacy and Safety of Tanreqing Injection for Pulmonary Infection in Patients with Tuberculosis: A Meta-Analysis.J Altern Complement Med. 2018 Nov;24(11):1051-1062. doi: 10.1089/acm.2018.0020. Epub 2018 Aug 20. J Altern Complement Med. 2018. PMID: 30124323
References
-
- Aliberti S, Goeminne PC, O’Donnell AE, et al. . Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10:298–306. - PubMed
-
- Chalmers JD, Polverino E, Crichton ML, et al. . Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med. 2023;11:637–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

