Optimizing CAR-T treatment: A T2EVOLVE guide to raw and starting material selection
- PMID: 39533710
- PMCID: PMC11897765
- DOI: 10.1016/j.ymthe.2024.11.017
Optimizing CAR-T treatment: A T2EVOLVE guide to raw and starting material selection
Abstract
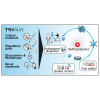
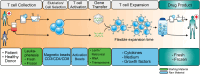
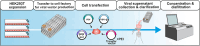
Chimeric antigen receptor (CAR)-T cell products, classified as Advanced Therapy Medicinal Products (ATMPs), have shown promising outcomes in cancer immunotherapy. The quality of raw and starting materials used in manufacturing is critical to ensure the efficacy and safety of CAR-T cell products and depends primarily on the selection of the right materials and the right suppliers. It is essential to consider a long-term strategy when selecting raw and starting materials to prevent delays in the supply of innovative, high-quality, and safe therapies to patients. A thorough assessment will allow developers not only to select suppliers who comply with regulatory requirements but also to ensure a sustainable supply of materials throughout the development and the commercial phases. A careful selection of materials and suppliers can avoid the need of comparability studies due to changes in the supply of materials, impacting costs and causing significant delays in development and treatment readiness for patients. This work, coordinated by the T2EVOLVE IMI consortium, provides guidance for the selection and handling of raw and starting materials. By following these suggestions, developers can ensure that they use high quality raw and starting materials through the product development and life cycle, resulting in safe and effective CAR-T therapies for patients.
Keywords: CAR-T cells; GMP; clinical trials; comparability; quality; raw material; stability; starting material; supplier selection; viral safety.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.M., S.A., and H.N. were employed by the Institut de Recherches Internationales Servier. T.T. and D.A. were employed by Astellas. R.C. was employed by Takeda Pharmaceuticals. I.S. was employed by Bayer Vital GmbH. B.S. was employed by Miltenyi Biotec B.V. & Co. KG.
Figures
References
-
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. (p. 133–188). https://eur-lex.europa.eu/eli/dir/2001/83/2022-01-01.
-
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia Ph. Eur. 5.2.12: raw materials of biological origin for the production of cell based and gene therapy medicinal products. https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition
-
- Belcastro L. Raw material qualification and optimization: the Janssen approach. Cell Gene Ther. Insights. 2020;6:235–240.
-
- Belcastro L., Brabec M., Clarke L., Luke R., Tomtishen III J.P. Fitting product to process: raw materials customization for cell therapy manufacturing success. Cell Gene Ther. Insights. 2021;7:183–198.
-
- Gee A.P. GMP CAR-T cell production. Best practice & research. Clin. Haematol. 2018;31:126–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

