Prevalence and Significance of AGR2 Expression in Human Cancer
- PMID: 39533806
- PMCID: PMC11557986
- DOI: 10.1002/cam4.70407
Prevalence and Significance of AGR2 Expression in Human Cancer
Abstract
Backround: Anterior gradient 2 (AGR2) is a resident endoplasmic reticulum (ER) protein with a vital role in embryonal development, mucus maturation, tissue regeneration, and wound healing.
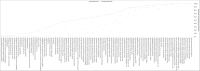
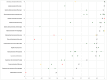
Methods: To determine the prevalence and clinical significance of AGR2 expression in cancer, a tissue microarray containing 14,966 tumors from 134 different tumor types and subtypes as well as 608 samples of 76 different normal tissue types was analyzed by immunohistochemistry (IHC).
Results: AGR2 positivity was found in 103 of 134 tumor categories, and 83 tumor categories contained at least one strongly positive case. AGR2 expression was most frequently seen in tumors of the female genital tract, particularly adenocarcinomas (up to 100%), various breast cancer subtypes (57.1%-100%), urothelial carcinoma (74.6%-100%), adenocarcinomas of the upper and lower gastrointestinal tract (93.6%-99.6%), and pancreaticobiliary cancers (65.2%-98.2%). AGR2 positivity was slightly less common in squamous cell carcinomas (46.4%-77.3%) and mainly absent in mesenchymal and lymphoid tumors. While AGR2 expression was only weak or absent in the normal thyroid, it was moderate to strong in 46.0% of adenomas, 52.8% of follicular carcinomas, and 81.8% of papillary carcinomas of the thyroid. High AGR2 expression was strongly linked to poor ISUP (p < 0.0001), Fuhrman (p < 0.0001), and Thoenes (p < 0.0001) grades as well as advanced pT stage (p = 0.0035) in clear cell renal cell carcinoma (ccRCC). Low AGR2 expression was associated with high BRE grade in breast cancer (p = 0.0049), nodal metastasis (p = 0.0275) and RAS mutation (p = 0.0136) in colorectal cancer, nodal metastasis (p = 0.0482) in endometrioid endometrial carcinoma, high grade in noninvasive urothelial carcinoma (p = 0.0003), and invasive tumor growth in urothelial carcinoma (p < 0.0001).
Conclusions: It is concluded that AGR2 expression occurs in a broad range of different tumor entities and that AGR2 assessment may serve as a diagnostic aid for the distinction of thyroidal neoplasms and as a prognostic marker in various cancer types.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The AGR2 antibody clone HMV‐325 was provided by ardoci GmbH (owned by a family member of G.S.).
Figures


References
-
- Aberger F., Weidinger G., Grunz H., and Richter K., “Anterior Specification of Embryonic Ectoderm: The Role of the Xenopus Cement Gland‐Specific Gene XAG‐2,” Mechanisms of Development 72 (1998): 115–130. - PubMed
-
- Zhu Q., Mangukiya H. B., Mashausi D. S., et al., “Anterior Gradient 2 Is Induced in Cutaneous Wound and Promotes Wound Healing Through Its Adhesion Domain,” FEBS Journal 284 (2017): 2856–2869. - PubMed
-
- Tereshina M. B., Ivanova A. S., Eroshkin F. M., et al., “Agr2‐Interacting Prod1‐Like Protein Tfp4 From Xenopus laevis Is Necessary for Early Forebrain and Eye Development as Well as for the Tadpole Appendage Regeneration,” Genes N. Y. N 2000 57 (2019): e23293. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

