Efficacy and Safety of Automated Insulin Delivery Systems in Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- PMID: 39533812
- PMCID: PMC11960199
- DOI: 10.4093/dmj.2024.0130
Efficacy and Safety of Automated Insulin Delivery Systems in Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Abstract
Backgruound: Automated insulin delivery (AID) systems studies are upsurging, half of which were published in the last 5 years. We aimed to evaluate the efficacy and safety of AID systems in patients with type 1 diabetes mellitus (T1DM).
Methods: We searched PubMed, Embase, Cochrane Library, Web of Science, and ClinicalTrials.gov until August 31, 2023. Randomized clinical trials that compared AID systems with other insulin-based treatments in patients with T1DM were considered eligible. Studies characteristics and glycemic metrics was extracted by three researchers independently.
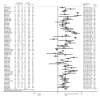
Results: Sixty-five trials (3,623 patients) were included. The percentage of time in range (TIR) was 11.74% (95% confidence interval [CI], 9.37 to 14.12; P<0.001) higher with AID systems compared with control treatments. Patients on AID systems had more pronounced improvement of time below range when diabetes duration was more than 20 years (-1.80% vs. -0.86%, P=0.031) and baseline glycosylated hemoglobin lower than 7.5% (-1.93% vs. -0.87%, P=0.033). Dual-hormone full closed-loop systems revealed a greater improvement in TIR compared with hybrid closed-loop systems (-19.64% vs. -10.87%). Notably, glycemia risk index (GRI) (-3.74; 95% CI, -6.34 to -1.14; P<0.01) was also improved with AID therapy.
Conclusion: AID systems showed significant advantages compared to other insulin-based treatments in improving glucose control represented by TIR and GRI in patients with T1DM, with more favorable effect in euglycemia by dual-hormone full closedloop systems as well as less hypoglycemia for patients who are within target for glycemic control and have longer diabetes duration.
Keywords: Diabetes mellitus, type 1; Insulin infusion systems; Meta-analysis; Systematic review.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


Comment in
-
Critical Insights into the Efficacy and Safety of Automated Insulin Delivery Systems in Patients with Type 1 Diabetes Mellitus (Diabetes Metab J 2025;49:235-51).Diabetes Metab J. 2025 May;49(3):516-517. doi: 10.4093/dmj.2025.0243. Epub 2025 May 1. Diabetes Metab J. 2025. PMID: 40367994 Free PMC article. No abstract available.
Similar articles
-
Efficacy of automated insulin delivery in pregnant women with type 1 diabetes: a meta-analysis and trial sequential analysis of randomized controlled trials.Acta Diabetol. 2024 Jul;61(7):831-840. doi: 10.1007/s00592-024-02284-3. Epub 2024 May 3. Acta Diabetol. 2024. PMID: 38700546 Review.
-
Real-world glycaemic outcomes of automated insulin delivery in type 1 diabetes: A meta-analysis.Diabetes Obes Metab. 2024 Sep;26(9):3753-3763. doi: 10.1111/dom.15718. Epub 2024 Jun 18. Diabetes Obes Metab. 2024. PMID: 38888056
-
Hybrid Closed-Loop Versus Manual Insulin Delivery in Adults With Type 1 Diabetes: A Post Hoc Analysis Using the Glycemia Risk Index.J Diabetes Sci Technol. 2024 Jul;18(4):764-770. doi: 10.1177/19322968241231307. Epub 2024 Feb 19. J Diabetes Sci Technol. 2024. PMID: 38372246 Free PMC article. Clinical Trial.
-
A comparison of ultra-rapid and rapid insulin in automated insulin delivery for type 1 diabetes: A systematic review and meta-analysis of randomized controlled trials.Diabetes Obes Metab. 2025 May;27(5):2658-2669. doi: 10.1111/dom.16268. Epub 2025 Feb 25. Diabetes Obes Metab. 2025. PMID: 39996365
-
Clinical evidence for high-risk CE-marked medical devices for glucose management: A systematic review and meta-analysis.Diabetes Obes Metab. 2024 Oct;26(10):4753-4766. doi: 10.1111/dom.15849. Epub 2024 Aug 14. Diabetes Obes Metab. 2024. PMID: 39143655
Cited by
-
Advances in Continuous Glucose Monitoring: Clinical Applications.Endocrinol Metab (Seoul). 2025 Apr;40(2):161-173. doi: 10.3803/EnM.2025.2370. Epub 2025 Apr 8. Endocrinol Metab (Seoul). 2025. PMID: 40195726 Free PMC article. Review.
-
Critical Insights into the Efficacy and Safety of Automated Insulin Delivery Systems in Patients with Type 1 Diabetes Mellitus (Diabetes Metab J 2025;49:235-51).Diabetes Metab J. 2025 May;49(3):516-517. doi: 10.4093/dmj.2025.0243. Epub 2025 May 1. Diabetes Metab J. 2025. PMID: 40367994 Free PMC article. No abstract available.
-
Efficacy and Safety of Automated Insulin Delivery Systems in Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis (Diabetes Metab J 2025;49:235-51).Diabetes Metab J. 2025 May;49(3):520-521. doi: 10.4093/dmj.2025.0282. Epub 2025 May 1. Diabetes Metab J. 2025. PMID: 40367992 Free PMC article. No abstract available.
References
-
- Gregory GA, Robinson TI, Linklater SE, Wang F, Colagiuri S, de Beaufort C, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10:741–60. - PubMed
-
- Alotaibi A, Al Khalifah R, McAssey K. The efficacy and safety of insulin pump therapy with predictive low glucose suspend feature in decreasing hypoglycemia in children with type 1 diabetes mellitus: a systematic review and meta-analysis. Pediatr Diabetes. 2020;21:1256–67. - PubMed
-
- Bosi E, Choudhary P, de Valk HW, Lablanche S, Castaneda J, de Portu S, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:462–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

